Overview
The primary focus of the article titled "10 Key Insights on Clinical Trial Innovation in Paraguay" is to underscore the innovative practices and advancements in clinical trials being implemented by bioaccess® in Paraguay. This article delineates various strategies, including:
- The adoption of decentralized trials
- The integration of artificial intelligence
- Regulatory compliance
- Patient-centric designs
These strategies collectively enhance the efficiency, accessibility, and effectiveness of clinical research in the region.
Introduction
In recent years, Paraguay has emerged as a beacon of clinical trial innovation, largely due to the pioneering efforts of bioaccess®. This organization stands at the forefront of transforming the landscape of clinical research, offering tailored solutions that enhance the efficiency and quality of studies across the Medtech sector.
With a rich history of over 15 years, bioaccess® is not only bridging the gap between groundbreaking medical technologies and effective clinical research but is also positioning Paraguay as an attractive hub for international collaborations and investments.
As the clinical trial environment evolves, the integration of digital health technologies, artificial intelligence, and patient-centric designs is reshaping the way trials are conducted, ensuring they are more accessible, efficient, and representative.
This article delves into the multifaceted advancements spearheaded by bioaccess®, exploring how these innovations are setting new standards in clinical research and contributing to the broader Medtech landscape in Latin America.
bioaccess: Pioneering Clinical Trial Innovation in Paraguay
bioaccess® has established itself as a leader in clinical trial innovation in Paraguay, dedicated to enhancing the effectiveness and quality of medical investigations. With over 15 years of specialized experience in the Medtech field, bioaccess® offers tailored solutions for a variety of medical studies, including:
- Early feasibility
- First-in-human trials
- Pivotal assessments
- Post-market follow-up research
This commitment to bridging the gap between innovative medical technologies and successful research has positioned Paraguay as an attractive destination for Medtech studies, fostering global partnerships and investments.
Recent advancements in research activities within Paraguay have been significant, with the nation benefiting from a flexible exchange rate system that enhances its economic stability and appeal for foreign investment. This system accommodates fluctuations in the national currency rate, providing advantages for Medtech companies considering investment in the region. However, it is crucial to acknowledge that there are currently two ongoing investor-state disputes involving U.S. companies concerning delayed government payments, which could influence the investment landscape.
The Pacific Alliance, comprising Colombia, Mexico, Chile, and Peru, further facilitates economic integration, creating opportunities for Medtech companies to flourish in the region. Paraguay's role within this alliance is essential, positioning the country to benefit from enhanced trade and investment in the medical technology sector, establishing it as a key player in Latin America.
As research innovation continues to evolve, bioaccess® remains at the forefront, utilizing its expertise to streamline processes such as:
- Site feasibility
- Investigator selection
- Study setup
- Start-up
- Regulatory compliance
- Project management
Julio Martinez-Clark observes that Medtech companies are increasingly pursuing expedited regulatory approval, ease of patient recruitment, and cost efficiency. The organization’s focus on these priorities aligns with the growing demand for effective medical studies, establishing it as a vital ally for Medtech firms navigating the complexities of the sector. Through its innovative approach, bioaccess® is not only advancing clinical trial innovation in Paraguay but also contributing to the broader field of Medtech innovation throughout Latin America.

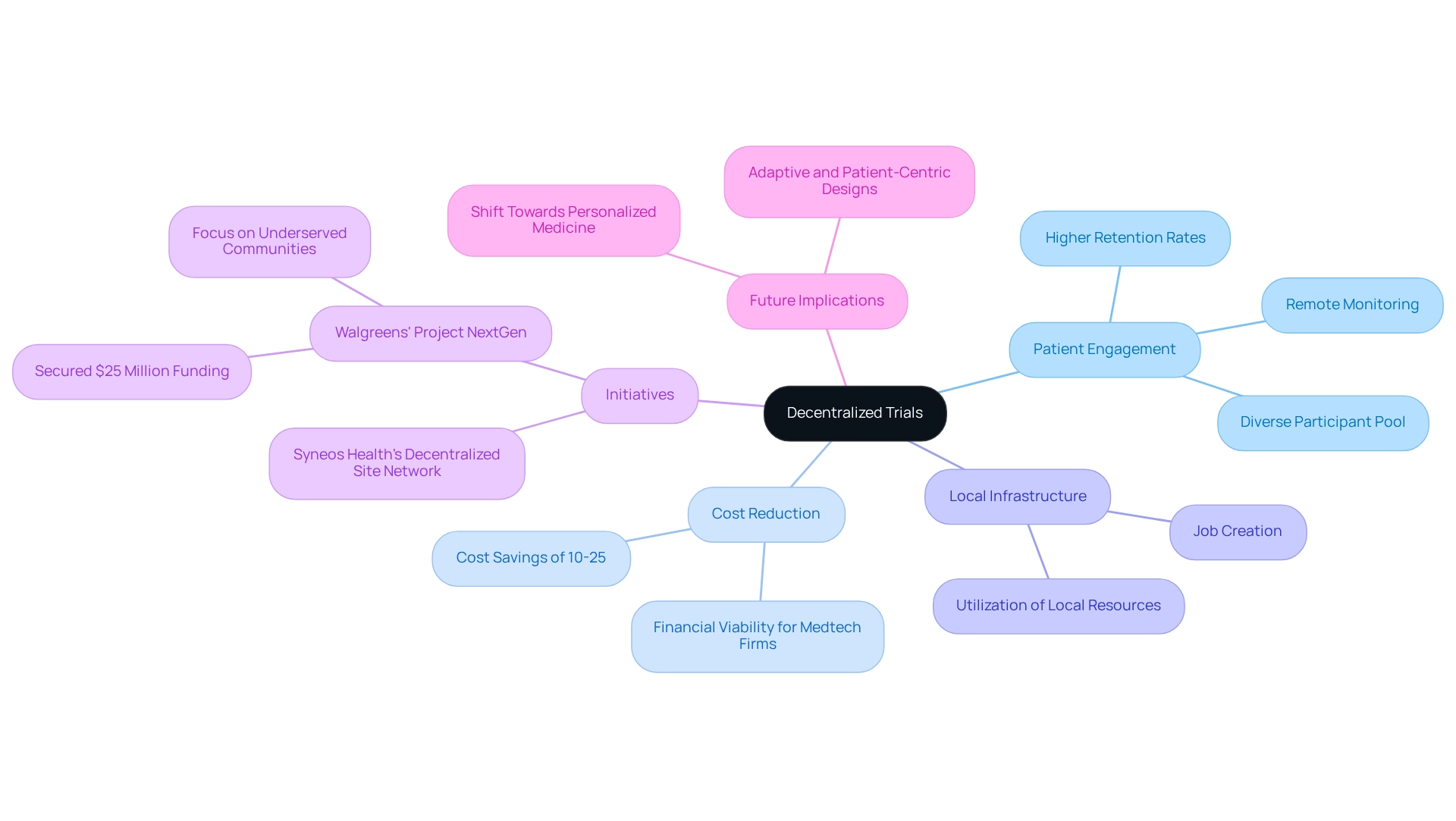
Decentralized Trials: Enhancing Patient Engagement and Accessibility
Decentralized studies are revolutionizing medical research by enabling patients to participate from the comfort of their homes, significantly lowering barriers to access. This innovative model enhances patient engagement through digital tools that facilitate remote monitoring and data collection.
In Paraguay, bioaccess has played a pivotal role in advancing clinical trial innovation by adopting Decentralized Clinical Trials (DCTs) and leveraging over 20 years of experience in managing research studies, including Early-Feasibility Studies and First-In-Human Studies, to effectively utilize local infrastructure. This approach allows for patient involvement without the burden of frequent site visits, resulting in higher retention rates and fostering a more diverse participant pool, which is crucial for the reliability of research studies.
Statistics indicate that clinical trial innovation in Paraguay, specifically through DCTs, can reduce costs by 10-25%, making them a financially viable option for Medtech firms, where cost efficiency is essential for successful clinical research. Furthermore, initiatives like Walgreens' Project NextGen, which secured $25 million in funding for decentralized studies, aim to enhance participation, particularly among underserved communities, potentially serving as a model for clinical trial innovation in Paraguay.
As Laura Wood, Senior Press Manager, notes, 'The shift towards personalized medicine, which often necessitates adaptive and patient-centric study designs, further propels the adoption of DCTs.' Additionally, Syneos Health's establishment of a decentralized research site network underscores the growing commitment to DCT adoption across Latin America.
As the landscape of medical research evolves, the benefits of DCTs in improving accessibility and participation are becoming increasingly evident, supported by bioaccess's comprehensive management services that contribute to job creation and healthcare improvement in local economies.
Artificial Intelligence: Revolutionizing Data Analysis in Clinical Trials
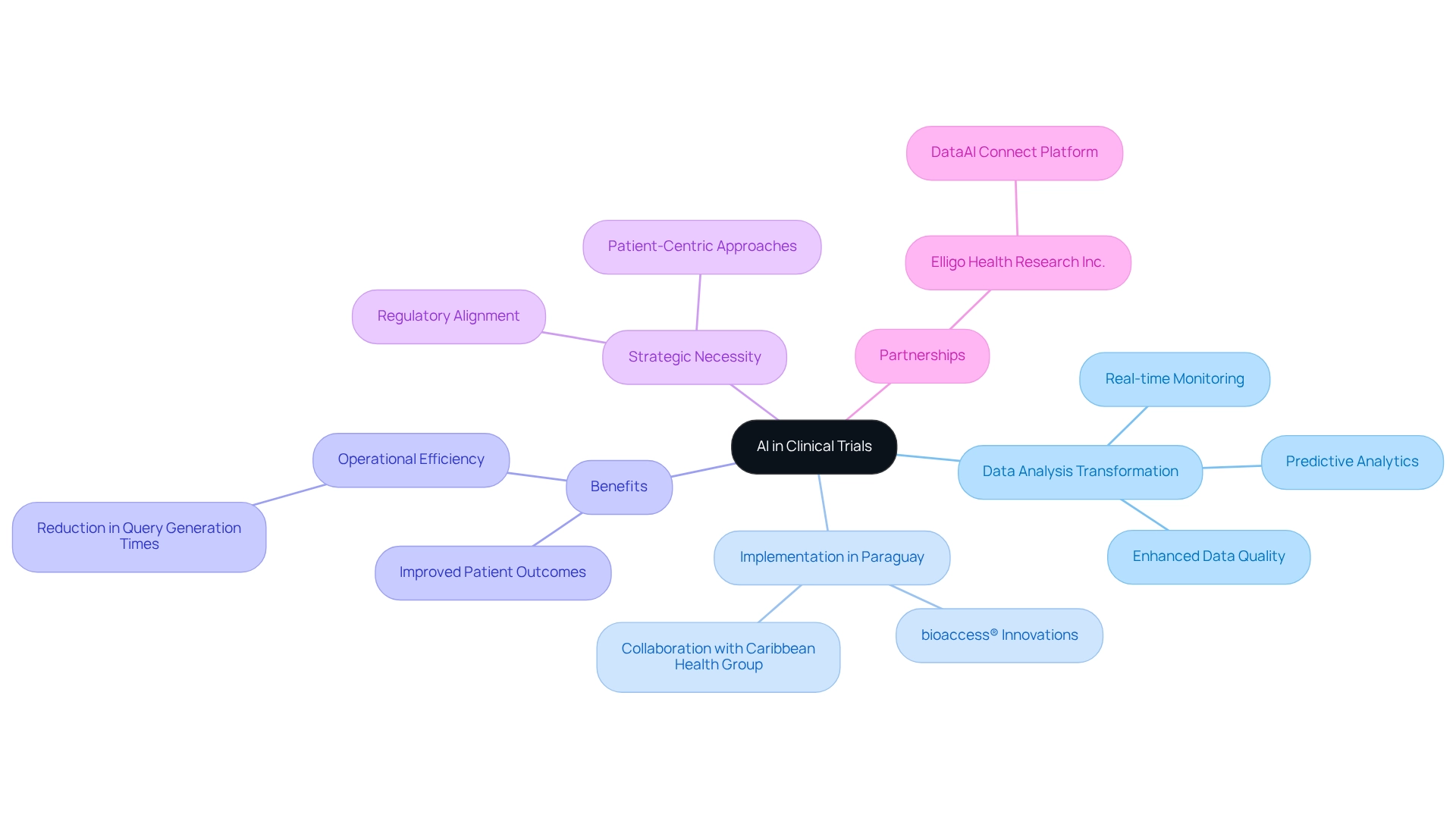
Artificial Intelligence (AI) is fundamentally transforming information analysis in medical studies, enabling the swift and accurate handling of extensive datasets. In Paraguay, bioaccess® is at the forefront of clinical trial innovation by integrating AI technologies into medical research, facilitating real-time information monitoring and predictive analytics. This integration significantly enhances data quality, allowing researchers to identify trends and potential issues early in the testing process. Consequently, decision-making becomes more informed, ultimately improving patient outcomes.
The implementation of AI in medical studies is not merely a trend; it is a strategic necessity that aligns with the growing regulatory demands and patient-centered approaches in the sector. By 2025, the incorporation of AI in healthcare studies is expected to optimize operations, reduce query generation times by up to 90%, as highlighted by Saama's Smart Data Quality, and foster a more effective trial environment. This evolution underscores the crucial role of clinical trial innovation in Paraguay in advancing healthcare methodologies and outcomes across Latin America.
Furthermore, Dr. Sergio Alvarado, Clinical Trial Manager at bioaccess™, emphasizes the importance of innovative medical studies and AI in diagnosing health issues, stating, 'AI is not merely a tool; it is a partner in enhancing our understanding of patient needs and improving outcomes.'
The collaboration between bioaccess™ and Caribbean Health Group to establish Barranquilla as a premier location for medical studies is further supported by Colombia's Minister of Health, highlighting the strategic initiatives aimed at enhancing health investigations in the region. As Elligo Health Research Inc. articulates, 'DataAI Connect, a groundbreaking data and technology platform, enables swift, data-informed studies,' showcasing the innovative dimensions of AI in this field.
By addressing the specific challenges faced in research studies, AI not only enhances operational efficiency but also aligns with the industry's regulatory and patient-focused requirements.
Regulatory Compliance: Ensuring Integrity in Clinical Research
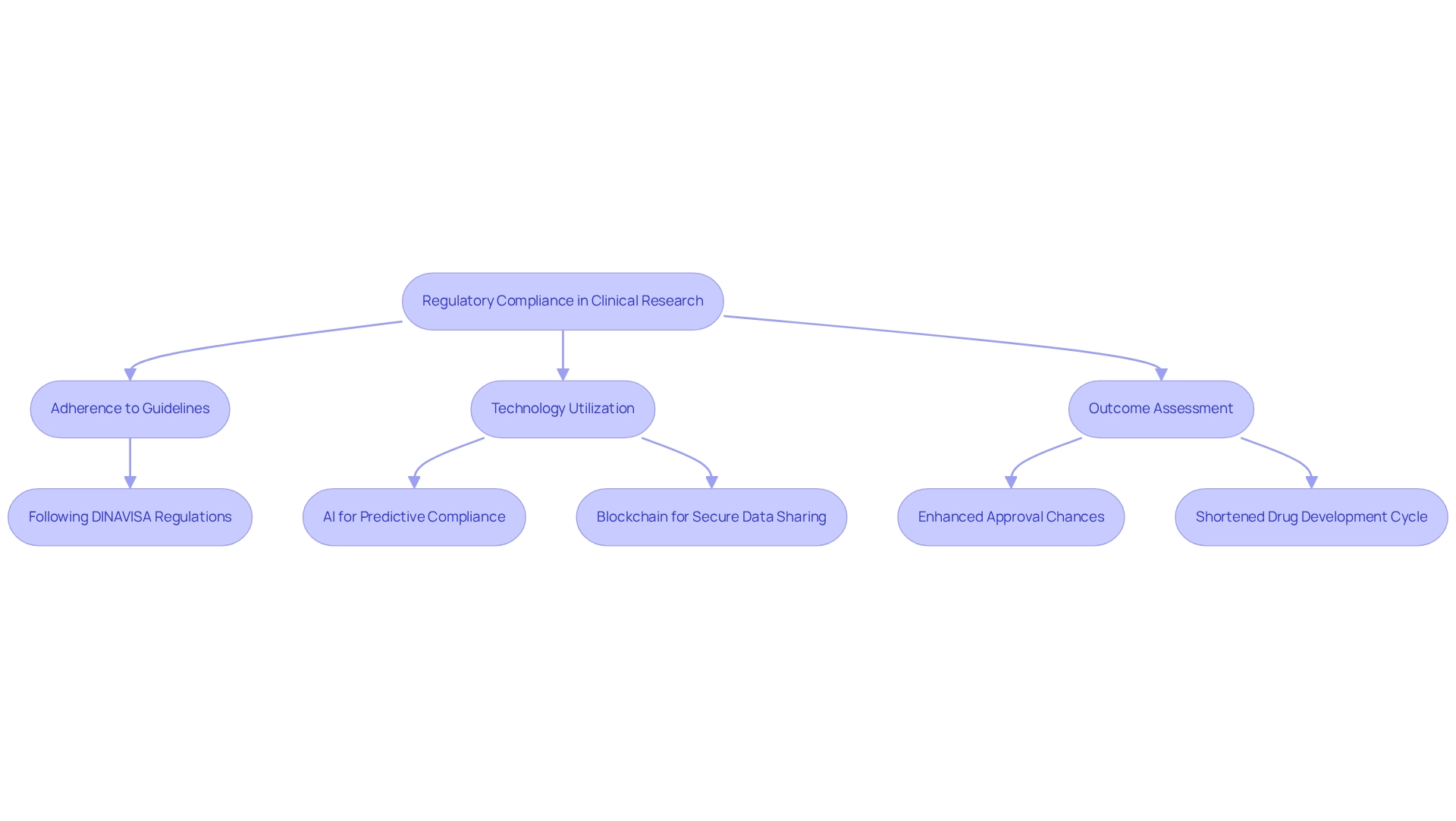
Regulatory compliance is essential in clinical research, serving as the foundation for ensuring study integrity and participant safety. In Paraguay, clinical trial innovation is exemplified by bioaccess®'s rigorous adherence to the stringent guidelines established by DINAVISA, the national regulatory authority. This unwavering commitment to adherence not only safeguards participant welfare but also enhances the trustworthiness of the information generated. By aligning with global standards, bioaccess® fosters trust among stakeholders, which is crucial for facilitating smoother approval processes. This proactive approach significantly accelerates the pathway to market for innovative medical devices.
To enhance information security and client confidence, bioaccess® has implemented comprehensive grievance and information protection procedures. Clients can address any concerns regarding the processing of their information by contacting our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com. We are dedicated to tackling these issues in accordance with relevant legislation, ensuring transparency and adherence throughout the research process.
The significance of regulatory compliance is underscored by recent statistics indicating that pharmaceutical firms adopting comprehensive information management solutions can shorten their drug development cycle by an average of 1.5 years, while also enhancing their chances of regulatory approval by 23%. Data management applications represent the largest segment of the clinical trial technology market, highlighting their critical role in ensuring compliance. Moreover, addressing information silos is vital, as they adversely impact research efficiency, making effective information management solutions essential for overcoming these challenges.
Utilizing advanced technologies like AI and blockchain enhances compliance monitoring, ensuring information integrity through secure sharing and immutable records. The case study titled "Leveraging Technology for Compliance Monitoring" illustrates how AI can improve predictive compliance risk assessment, while blockchain facilitates secure data sharing, thereby enhancing overall compliance management. These innovations not only streamline compliance procedures but also bolster the integrity of medical studies.
As emphasized by industry specialist Federico Waisberg, "Real-world evidence may signify a unique opportunity to improve local studies," further underscoring the necessity for robust regulatory frameworks. In 2025, the emphasis on regulatory adherence in clinical trial innovation in Paraguay will be paramount, as it directly influences the integrity of research results and the advancement of medical technologies. Bioaccess® guarantees compliance with DINAVISA regulations through meticulous planning and implementation of research studies, thereby reinforcing its position within the regulatory landscape.
Collaborative Approaches: Fostering Innovation in Clinical Trials
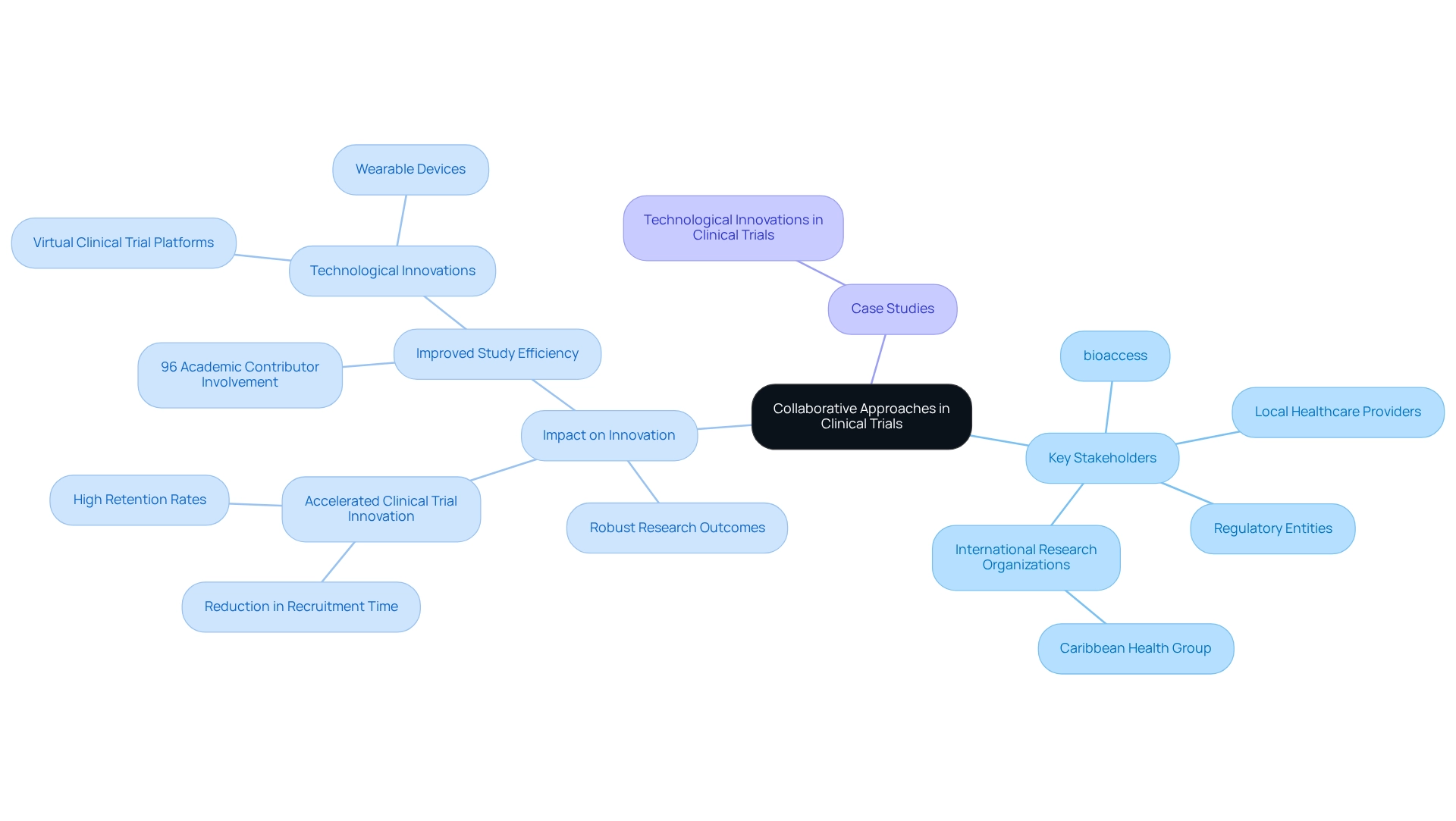
Team-oriented methods are essential for fostering innovation in medical studies. bioaccess actively collaborates with local healthcare providers, regulatory entities, and international research organizations, such as Caribbean Health Group, to create a synergistic environment for medical research. This partnership, supported by Colombia's Minister of Health, aims to position Barranquilla as a premier hub for medical studies in Latin America.
By leveraging the expertise and resources of diverse stakeholders, these collaborations enhance the planning and execution of clinical studies, leading to more robust research outcomes and accelerating clinical trial innovation in Paraguay. In 2025, statistics reveal that 96% of studies had the corresponding author as an academic contributor, underscoring the pivotal role of collaboration in improving study efficiency.
Case studies, such as 'Technological Innovations in Clinical Studies,' demonstrate the effectiveness of these collaborative efforts, showcasing how innovative study designs—including virtual platforms and wearable devices—optimize data collection and elevate patient engagement. Moreover, partnerships with organizations like IDx Technologies and GlobalCare Clinical Trials have contributed to clinical trial innovation in Paraguay, resulting in over a 50% reduction in recruitment time and impressive 95% retention rates.
By nurturing these collaborations, bioaccess is at the forefront of transforming health studies in the region, ensuring that the potential of medical devices is realized more swiftly and efficiently. As Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, reflects on his experience with bioaccess during its initial human study in Colombia, the commitment to participant safety remains a top priority, even in the face of the inherent risks associated with medical research.
Patient-Centric Designs: Prioritizing Participant Experience
Patient-focused designs have become increasingly recognized as essential for enhancing participant experiences in clinical studies. By prioritizing patient feedback in research protocols, bioaccess ensures that studies are tailored to meet the needs of participants. This strategy not only boosts recruitment and retention rates but also significantly enhances the quality of data collected.
As Don Berwick, former Administrator of the Centers for Medicare and Medicaid Services, aptly stated, "Listening to patients is the cornerstone of patient-centered care." When participants feel their needs are acknowledged, they are more likely to engage actively in the study, leading to more reliable outcomes.
In 2025, statistics indicate that studies incorporating patient-focused designs have seen a notable increase in enrollment success, underscoring the effectiveness of this approach. Furthermore, a collaboration initiated in March 2024 between bioaccess and GlobalCare Clinical Trials exemplifies the growing emphasis on patient-centered methodologies in medical studies, achieving over a 50% reduction in recruitment time and 95% retention rates.
Case studies, such as "Safety and Risks in Clinical Trials," illustrate that rigorous attention to participant safety and ethical standards fosters trust, which is vital for the successful execution of trials. Industry specialists emphasize that integrating compassion and understanding into medical studies is crucial for achieving significant outcomes and advancing medical technology, particularly in Paraguay, where clinical trial innovation in Paraguay is increasingly focused on patient-centered designs.
Real-World Evidence: Bridging Research and Clinical Practice
Real-world evidence (RWE) is increasingly recognized as a pivotal component in medical studies, offering essential insights into the performance of medical products in everyday settings. bioaccess® effectively leverages RWE to bridge the gap between research studies and medical practice, ensuring that study results translate into real-world applicability. By integrating RWE into their studies, bioaccess® amplifies the significance of their investigations, which is crucial for informed healthcare decisions and improved patient outcomes.
The importance of RWE is underscored by the challenges faced in study participation, particularly in oncology and chronic diseases, where low enrollment rates highlight the need for improved access and eligibility criteria. A case study titled "Challenges in Clinical Trial Participation" illustrates these issues, emphasizing the necessity of addressing these challenges to ensure that new treatments reach diverse populations and advance research in critical health domains.
In 2025, the application of RWE in medical studies is expected to expand, particularly in Paraguay, where regulatory frameworks are evolving to support clinical trial innovation in Paraguay. This shift is driven by the recognition that RWE can enhance study designs, ultimately increasing the likelihood of success. As noted, "By integrating RWE, biotech consulting companies and pharma consulting firms can enhance their trial designs, ultimately boosting the chances of success." Statistics indicate that RWE is gaining traction as a form of evidence across the industry, reinforcing its importance in the Medtech sector.
As bioaccess® continues to innovate in medical research, the incorporation of RWE not only elevates the quality of their studies but also aligns with the broader objective of enhancing healthcare outcomes through evidence-based practices. The collaboration between bioaccess™ and Caribbean Health Group to position Barranquilla as a premier site for medical studies in Latin America, supported by Colombia's Minister of Health, exemplifies this commitment. Furthermore, bioaccess®'s dedication to ensuring information security and client confidence through robust protective measures underscores their commitment to transparency and compliance in medical device evaluations. For further insights, the full event recording of the Life Sciences – In Focus series is available for viewing, showcasing ongoing discussions surrounding RWE and its implications in the industry. Additionally, the validation method for RWE tools and processes, guided by GAMP recommendations, ensures regulatory compliance, further emphasizing the significance of RWE in research studies.
Digital Health Technologies: Streamlining Clinical Trial Processes
Digital health technologies are revolutionizing research processes by facilitating remote data gathering, patient observation, and streamlined communication. bioaccess® harnesses these advancements to markedly enhance study efficiency and participant engagement. With over 20 years of experience in Medtech, bioaccess® offers comprehensive clinical study management services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Post-Market Clinical Follow-Up Studies
By leveraging mobile health applications and telemedicine solutions, bioaccess® empowers participants to access study information and submit data from the comfort of their homes. This approach not only reduces dropout rates but also improves the quality of information, ensuring that experiments are conducted more effectively.
In 2025, the integration of digital health technologies is expected to further boost trial efficiency, with remote information gathering becoming increasingly prevalent. Notably, around one-third of all IoT devices are now utilized within healthcare organizations, highlighting the growing reliance on technology in medical research. Additionally, the medical information interoperability market is projected to reach $6.2 billion by 2027, driven by a 12.9% CAGR, underscoring the critical role of seamless information exchange in enhancing patient outcomes.
Dr. Eric Topol aptly remarks, "The crowd will see you now, the patient will see you now, Dr. Google will see you now, the robot will see you now, the avatar will see you now. Everyone will see you now. Everyone but the doctor." This statement encapsulates the transformative influence of digital health technologies on patient engagement. By embracing these innovations, bioaccess® is strategically positioned to lead advancements in clinical trial innovation in Paraguay, ensuring they remain at the forefront of this evolving landscape.

Diversity and Inclusion: Enhancing Representativeness in Trials
Improving diversity and inclusion in clinical studies is essential for producing representative information that accurately reflects the wider population. In 2025, only 38% of North American studies reported participants' racial data, highlighting a significant gap in diversity that can adversely affect treatment efficacy across different demographics. This lack of representation can lead to treatments that are less effective for underrepresented groups, underscoring the urgent need for inclusive study practices.
In Paraguay, bioaccess® is committed to actively enlisting participants from diverse backgrounds, which is essential for advancing clinical trial innovation in Paraguay and ensuring that their studies are both inclusive and equitable. This focus on diversity not only enhances the generalizability of findings but also addresses critical health disparities, ultimately leading to more effective healthcare solutions for all populations.
The moral and scientific necessity to enhance diversity in medical studies is emphasized by specialists who assert that varied participant recruitment is vital for the reliability of study results. Furthermore, the partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a premier location for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative not only enhances patient access and participation but also contributes to local economic growth through job creation and improved healthcare services.
By implementing effective strategies such as community outreach, tailored recruitment efforts, and collaborations with local organizations, bioaccess® plays a crucial role in promoting medical devices and encouraging clinical trial innovation in Paraguay. Additionally, bioaccess® brings over 20 years of experience in overseeing various types of medical device research studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, ensuring that their evaluations are not only diverse but also scientifically rigorous.
Adaptive Trial Designs: Increasing Efficiency in Clinical Research
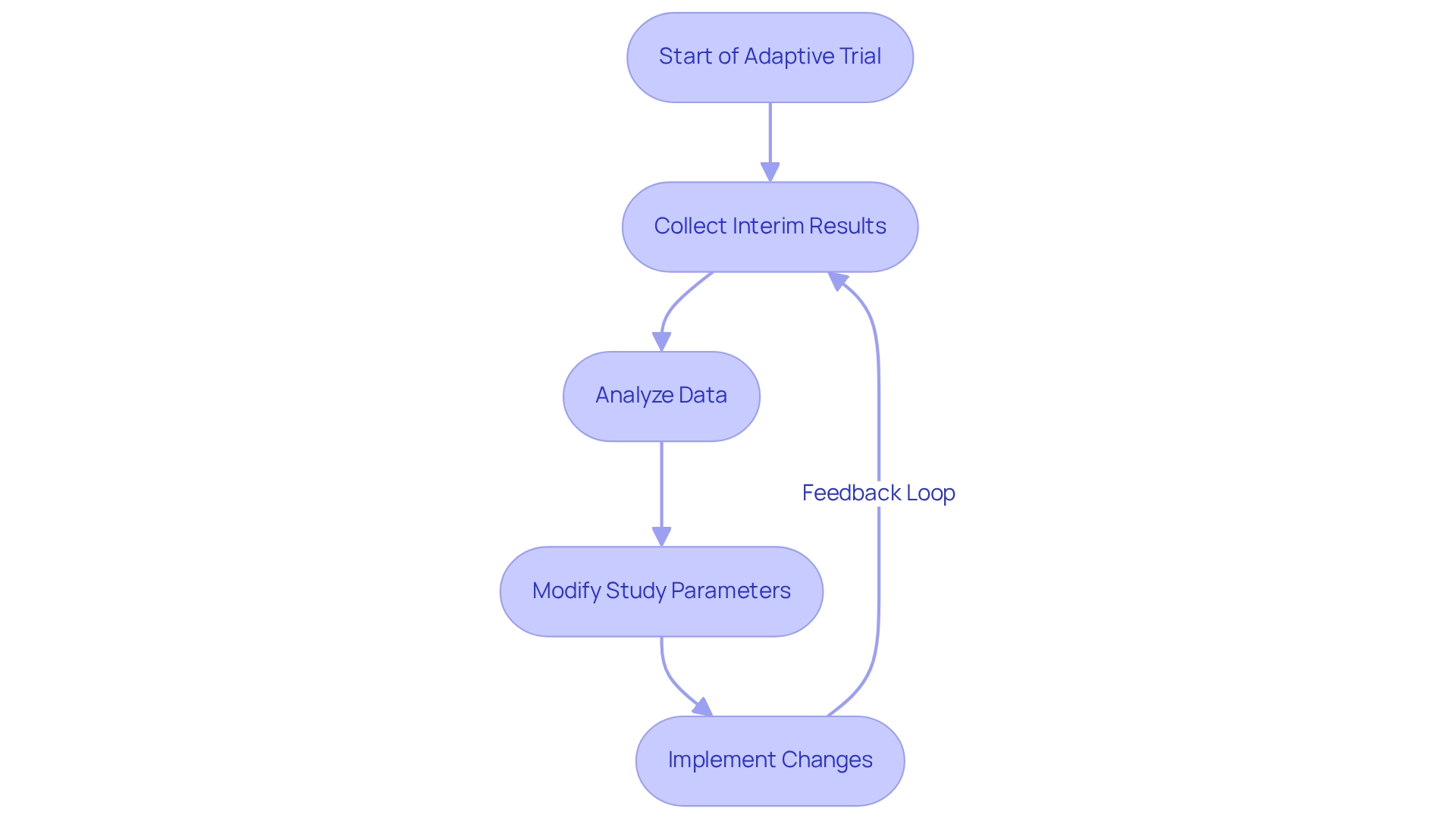
Adaptive study designs are gaining traction in medical research, particularly for their ability to modify study parameters based on interim results. This inherent flexibility enables researchers to make data-driven choices that significantly improve study efficiency and effectiveness. In 2025, it is estimated that around 20% of clinical studies in Latin America, particularly in oncology, will employ adaptive designs, indicating their increasing significance in the field. These designs optimize resource usage and enhance patient results by enabling studies to adjust rapidly to new information.
For example, seamless phase one and phase two study designs can enhance patient populations based on early treatment effects, resulting in more effective studies. At bioaccess®, with more than 20 years of expertise in Medtech, adaptive designs are utilized to enhance research studies, especially in early-feasibility and first-in-human investigations. This ensures that evaluations can shift rapidly in response to new insights, ultimately leading to quicker and more dependable results. A notable example is ReGelTec's Early Feasibility Study on HYDRAFIL™ for treating chronic low back pain in Colombia, where the procedures were successfully managed with remote oversight, showcasing bioaccess®'s commitment to innovation and regulatory excellence.
As Cyrus Mehta, Ph.D., observes, "Adaptive designs can potentially result in more efficient studies and improved patient outcomes." The advantages of adaptive studies are clear, as they enable a more flexible method in clinical investigation, making them a significant resource for clinical trial innovation in Paraguay and advancing medical technology in Latin America. Therefore, clinical research directors should advocate for the integration of adaptive trial designs in their studies to promote clinical trial innovation in Paraguay and improve patient care.
Conclusion
The innovations in clinical trials spearheaded by bioaccess® are positioning Paraguay as a key player in the Medtech sector. With over 15 years of experience, bioaccess® enhances the efficiency and quality of clinical research, attracting international collaborations and investments.
The integration of decentralized trials, artificial intelligence, and digital health technologies is transforming the clinical research landscape. By prioritizing patient-centric designs and utilizing real-world evidence, bioaccess® ensures that studies are more accessible and representative, leading to improved outcomes and increased participant trust. Their commitment to regulatory compliance and collaboration further bolsters the credibility of clinical research.
As the clinical trial environment evolves, the focus on diversity, adaptive trial designs, and digital health tools will be vital for the future of Medtech research in Latin America. Through these efforts, bioaccess® is fostering a more inclusive research environment and driving advancements in medical technology, ultimately providing better healthcare solutions for diverse populations.
In summary, bioaccess® is revolutionizing clinical trials in Paraguay, setting new standards for efficiency and innovation. By championing these advancements, it establishes Paraguay as a hub for clinical research excellence, contributing to improved global health outcomes. The future of clinical trials hinges on innovation and collaboration, with bioaccess® leading the way toward transformative possibilities in Medtech.
Frequently Asked Questions
What is bioaccess® and its role in clinical trial innovation in Paraguay?
Bioaccess® is a leader in clinical trial innovation in Paraguay, focusing on enhancing the effectiveness and quality of medical investigations. With over 15 years of experience in the Medtech field, it provides tailored solutions for various medical studies.
What types of medical studies does bioaccess® specialize in?
Bioaccess® specializes in several types of medical studies, including early feasibility studies, first-in-human trials, pivotal assessments, and post-market follow-up research.
How has Paraguay become an attractive destination for Medtech studies?
Paraguay has become an attractive destination for Medtech studies due to its commitment to bridging innovative medical technologies with successful research, along with a flexible exchange rate system that enhances economic stability and appeal for foreign investment.
What are the current challenges for investors in Paraguay?
There are currently two ongoing investor-state disputes involving U.S. companies concerning delayed government payments, which could impact the investment landscape in Paraguay.
What is the Pacific Alliance, and how does it relate to Paraguay?
The Pacific Alliance is an economic integration initiative comprising Colombia, Mexico, Chile, and Peru. Paraguay's role within this alliance is essential for enhancing trade and investment in the medical technology sector.
What processes does bioaccess® streamline in clinical trials?
Bioaccess® streamlines several processes in clinical trials, including site feasibility, investigator selection, study setup, start-up, regulatory compliance, and project management.
What trends are influencing Medtech companies in Paraguay?
Medtech companies are increasingly pursuing expedited regulatory approval, ease of patient recruitment, and cost efficiency, which aligns with the growing demand for effective medical studies.
How are Decentralized Clinical Trials (DCTs) changing medical research?
DCTs allow patients to participate in studies from home, lowering barriers to access and enhancing patient engagement through digital tools, resulting in higher retention rates and a more diverse participant pool.
What financial benefits do DCTs provide for Medtech firms?
DCTs can reduce costs by 10-25%, making them a financially viable option for Medtech firms focused on cost efficiency in clinical research.
How is bioaccess® integrating Artificial Intelligence (AI) into medical research?
Bioaccess® is integrating AI technologies into medical research to facilitate real-time information monitoring and predictive analytics, enhancing data quality and improving patient outcomes.
What are the expected impacts of AI on healthcare studies by 2025?
By 2025, the incorporation of AI in healthcare studies is expected to optimize operations and reduce query generation times by up to 90%, improving the overall trial environment.
What collaborations is bioaccess® involved in to enhance medical studies?
Bioaccess® collaborates with organizations like Caribbean Health Group to establish strategic initiatives aimed at enhancing health investigations in the region, including establishing Barranquilla as a key location for medical studies.




