Overview
This article presents strategies for conducting cost-efficient clinical trials in Peru, underscoring various methods to optimize expenses while ensuring high-quality outcomes. It highlights the significance of tailored research services, regulatory consulting, and innovative recruitment strategies. Each of these elements plays a crucial role in reducing costs and enhancing operational efficiency within the Medtech sector.
Introduction
In the rapidly evolving landscape of clinical research, the pursuit of cost-effective solutions has never been more critical. Organizations are increasingly turning to innovative strategies that not only streamline trial processes but also enhance patient engagement and improve outcomes. From leveraging advanced algorithms for patient matching to implementing virtual trials that reduce operational expenses, the Medtech sector is witnessing a transformation aimed at maximizing efficiency.
With a focus on tailored approaches and regulatory excellence, companies like bioaccess® are leading the charge in Latin America, ensuring that clinical trials are not only affordable but also ethically conducted. This article delves into the various methodologies and technologies being employed to revolutionize clinical trials, offering insights into how organizations can navigate the complexities of research while driving significant cost savings.
bioaccess: Accelerating Cost-Effective Clinical Trials in Peru
bioaccess® excels in delivering tailored research services that expedite the development of medical devices in Peru. With over 20 years of experience in the Medtech field, the organization leverages its extensive knowledge of local regulatory systems and operational efficiencies to assist Medtech firms in conducting early feasibility studies, first-in-human studies, pilot studies, and critical evaluations at significantly reduced costs.
Recent data indicates that cost-efficient clinical trials in Peru have an average expense that is substantially lower than in many other regions, making it an attractive option for Medtech firms. Key strategies include optimizing site selection and enhancing patient recruitment, both critical for minimizing study costs while ensuring high-quality outcomes.
As Konstantin Kalinin, Head of Content, states, "Compliance with standards like FDA or CE requires rigorous testing and documentation, which can be both time and resource-intensive." This customized approach not only accelerates the research process but also ensures adherence to stringent standards, ultimately leading to improved patient outcomes and operational efficiency.
Furthermore, bioaccess® provides comprehensive research study management services, including feasibility assessments, compliance evaluations, study setup, import permits, project oversight, and reporting, representing a significant advancement in research management.
To maximize cost efficiency, Medtech companies should consider implementing these strategies, such as conducting cost-efficient clinical trials in Peru, while continuously evaluating their testing processes for potential enhancements. For further insights, companies are encouraged to schedule a meeting with bioaccess® to explore customized solutions for their research needs.
CureMatch: Streamlining Clinical Trials with Intelligent Matching Solutions
CureMatch employs advanced algorithms to effectively connect patients with clinical studies tailored to their unique genetic profiles. This innovative matching process accelerates patient recruitment while significantly enhancing the likelihood of success by ensuring that the most suitable participants are enrolled. By optimizing this recruitment strategy, CureMatch minimizes both the time and costs typically associated with traditional methods. For example, studies indicate that diversified recruitment strategies—leveraging pharmacy and physician referrals alongside online campaigns—can improve enrollment rates and expand the participant pool. This multifaceted approach has proven efficient in achieving enrollment goals more effectively, ultimately facilitating quicker advancements in medical technology.
In the context of Latin America, particularly through the partnership between bioaccess™ and Caribbean Health Group, there is a concerted effort to position Barranquilla as a premier hub for research studies. Supported by Colombia's Minister of Health, this initiative aims to establish a robust framework for medical research that not only enhances patient recruitment but also stimulates local economies through job creation and healthcare improvements. Furthermore, involving diverse communities in observational studies may serve as a 'gateway' to increased research participation overall, underscoring the importance of inclusivity in recruitment strategies. To fully leverage the benefits of intelligent matching, research directors should contemplate implementing these strategies to boost patient engagement and streamline study processes.
Novotech: Pioneering Virtual Clinical Trials for Cost Efficiency
Virtual research studies (VCTs) are revolutionizing the field of medical investigation by enabling remote patient observation and data collection. This innovative approach eliminates the necessity for physical testing locations, resulting in substantial reductions in costs associated with site administration and patient transportation.
Studies reveal that VCTs can yield significant financial savings, particularly through decreased travel expenses and site infrastructure requirements. For example, VCTs can reduce costs by as much as 30%, making them an attractive option for research endeavors. By leveraging advanced technologies, VCTs not only boost patient engagement and retention but also markedly decrease operational expenses.
Furthermore, the integration of electronic patient-reported outcomes (ePROs) has proven to enhance data accuracy, thereby improving the overall quality of research findings. As Robert M. Califf, MD, FDA Commissioner, stated, "VCTs can enhance convenience for participants, reduce the burden on caregivers, expand access to more diverse populations, improve efficiencies, and facilitate research on rare diseases and conditions affecting populations with limited mobility."
The effective implementation of remote monitoring strategies will be crucial for maximizing the efficiency and effectiveness of medical studies within the Medtech sector. Additionally, attracting top digital talent is vital for the successful validation and utilization of these remote technologies.
With over 20 years of experience in Medtech, bioaccess® offers comprehensive study management services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Post-Market Follow-Up Studies
This expertise ensures that research directors can refine their study processes while navigating the unique challenges and opportunities present in the Latin American Medtech landscape, ultimately fostering global health advancement through international collaboration and innovation.
Emergo by UL: Regulatory Consulting for Affordable Clinical Trials
bioaccess plays a pivotal role in regulatory consulting, expertly guiding Medtech companies through the intricate approval processes in Peru. Their comprehensive service capabilities encompass:
- Feasibility studies
- Selection of research sites
- Principal investigator (PI) identification
- Thorough review and feedback on study documents to ensure compliance with local regulations
This strategic approach significantly mitigates the risk of costly delays and rejections. By providing valuable insights into regulatory submissions and compliance, bioaccess empowers companies to initiate cost-efficient clinical trials in Peru more swiftly and economically. This is especially critical in a landscape where the yearly expenditure on research studies in Latin America's Andean Region has surged from $3-4 million to over $50 million, reflecting a growing interest from biopharmaceutical companies. As the region enhances its research capabilities, effective regulatory consulting becomes essential for navigating approval processes efficiently, ultimately leading to cost-efficient clinical trials in Peru.
However, it is crucial to recognize that a very small number of approved research studies in Peru focus on neglected tropical diseases, highlighting a concerning lack of attention to health issues affecting vulnerable populations (Martin Javier Yagui). Additionally, cultural differences may lead patients in Latin America to agree to participate in research studies without fully discussing treatment options or risks with their physicians. By addressing these cultural and socio-economic barriers, regulatory consulting can play a vital role in ensuring that studies are not only efficient but also ethically conducted, thereby enhancing patient protection and quality in research endeavors.
Greenlight Guru: Ensuring Quality Management in Cost-Effective Trials
bioaccess® offers a comprehensive suite of clinical study coordination services tailored to the unique landscape of Latin America. Our expertise spans:
- Feasibility studies
- Meticulous site selection
- Compliance reviews to guarantee adherence to local regulations
- Thorough review and feedback on study documents to align with country requirements
- Management of the setup for experiments
- Oversight of the import permits for investigational devices
By optimizing project management and reporting processes, bioaccess® not only improves operational efficiency but also supports the implementation of cost-efficient clinical trials in Peru. This integrated approach not only fosters job creation and economic growth in local communities but also enhances healthcare outcomes through cutting-edge medical technologies.
As the leading Contract Research Organization in the region, bioaccess® is committed to advancing medical device studies with a strong focus on regulatory excellence and global collaboration. Leveraging our expertise, organizations can navigate the complexities of the Latin American Medtech environment, ensuring successful market entry and impactful research studies.
As the landscape of clinical research evolves, partnering with bioaccess® becomes essential for organizations aiming to conduct cost-efficient clinical trials in Peru and refine their study processes.
Precision for Medicine: Optimizing Clinical Trial Management for Cost Savings
bioaccess® leverages extensive expertise in study management to enhance operational efficiency and achieve significant cost reductions, particularly in the context of cost-efficient clinical trials in Peru. With more than 20 years of experience in the Medtech sector, bioaccess® specializes in a variety of studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This comprehensive approach to conducting cost-efficient clinical trials in Peru ensures optimal resource allocation and streamlined processes, which are critical for minimizing expenses. In collaboration with Caribbean Health Group, bioaccess® is dedicated to positioning Barranquilla as a leading hub for medical studies in Latin America, a move endorsed by Colombia's Minister of Health. This initiative aims to attract additional medical research projects to the region, thereby enhancing the overall landscape for studies.
By utilizing data analytics and real-time monitoring, bioaccess® not only improves efficiency in studies but also supports cost-efficient clinical trials in Peru by curtailing unnecessary costs. The integration of artificial intelligence in healthcare research data management has been shown to boost success rates by 10%, underscoring the significance of data-driven decision-making. Furthermore, real-time data access empowers research sites to effectively navigate the complexities of medical studies, facilitating informed decision-making that enhances patient outcomes and overall study efficiency.
Clinical Research Directors can implement similar real-time data solutions in their practices to improve study oversight. These advancements exemplify how optimizing medical studies through data analysis can lead to substantial cost savings and enhanced operational efficiency, particularly in the context of cost-efficient clinical trials in Peru. Additionally, organizations that embrace end-to-end data management solutions reduce their drug development timelines by an average of 1.5 years and improve their likelihood of regulatory approval by 23%, highlighting the crucial role of effective data management in achieving operational excellence.
To further enhance your research study oversight, consider integrating compliance evaluations and study setup services into your processes. This holistic strategy can significantly elevate your operational efficiency and support the implementation of cost-efficient clinical trials in Peru.
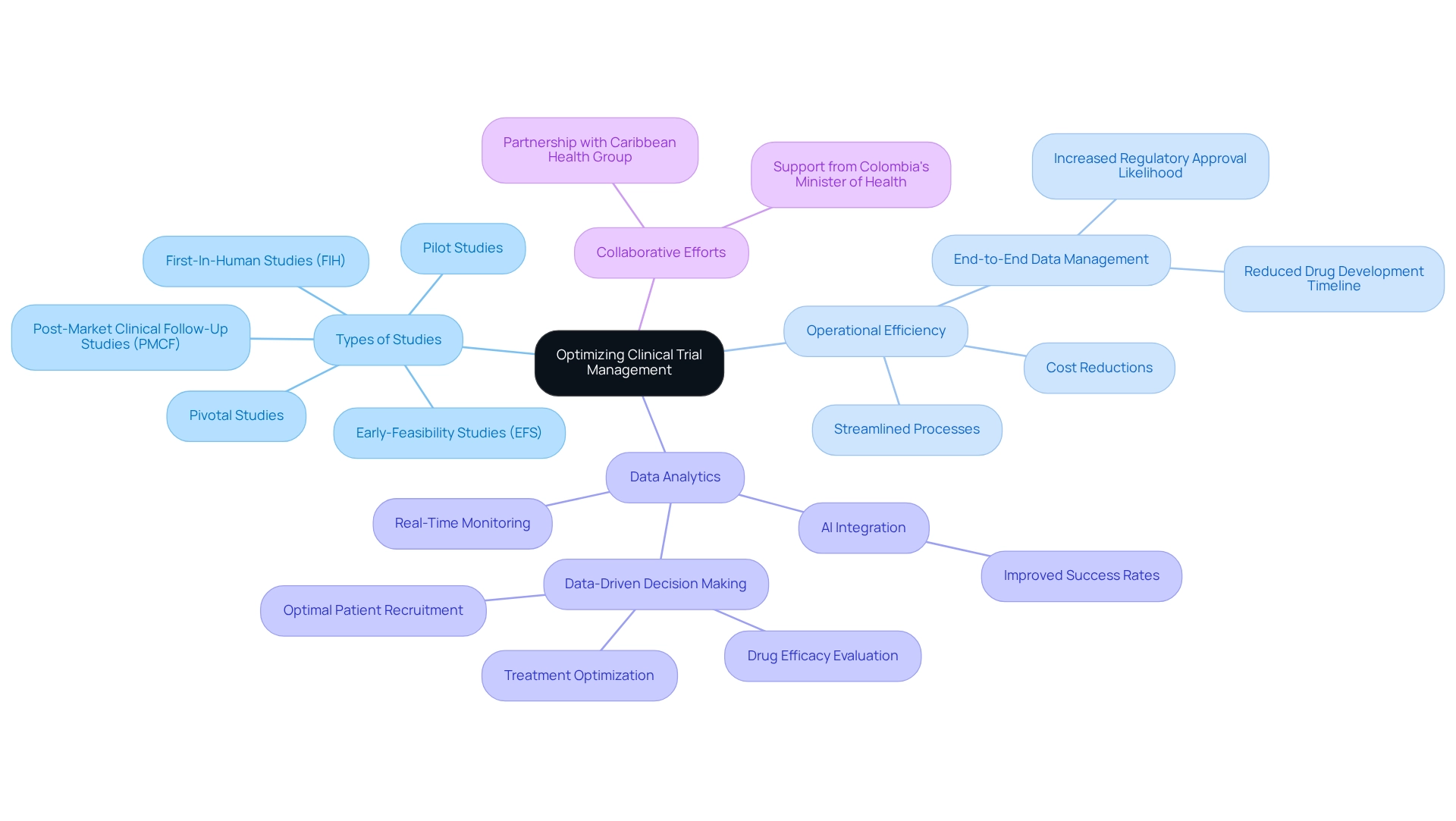
QbD Group: Tailored Solutions for Cost-Effective Medical Device Trials
Bioaccess excels in providing tailored solutions for medical device studies, emphasizing careful planning and robust risk oversight strategies. Their customized approach aims to reduce expenses while ensuring compliance and maintaining high-quality standards. By addressing the unique needs of each study, bioaccess not only assists clients in achieving their goals efficiently but also plays a crucial role in facilitating cost-efficient clinical trials in Peru, significantly alleviating the financial burden associated with research.
Statistics indicate that the expense of site data verification (SDV) constitutes between 0.9 to 1.6 percent of total study expenses, underscoring the importance of effective risk oversight in managing costs. Furthermore, a recent analysis of 3,410 protocols from 17 pharmaceutical and biotechnology firms revealed an astonishing 3,596 amendments and 19,345 modifications, highlighting the complexities and potential inefficiencies in study management.
In light of these challenges, bioaccess's success stories in risk management demonstrate how integrating healthcare and research datasets can enhance efficiency, despite the obstacles presented by incompatible systems. This integration not only streamlines processes but also fosters a more inclusive approach to medical research, as engaging diverse communities in observational studies may serve as a 'gateway' to increased research participation overall.
By leveraging their expertise, bioaccess improves the overall efficiency of medical device development in Latin America, addressing the rising costs associated with drug development, which, as of 2023, average around $2.6 billion in the US. This reality highlights the necessity for innovative strategies, such as cost-efficient clinical trials in Peru.
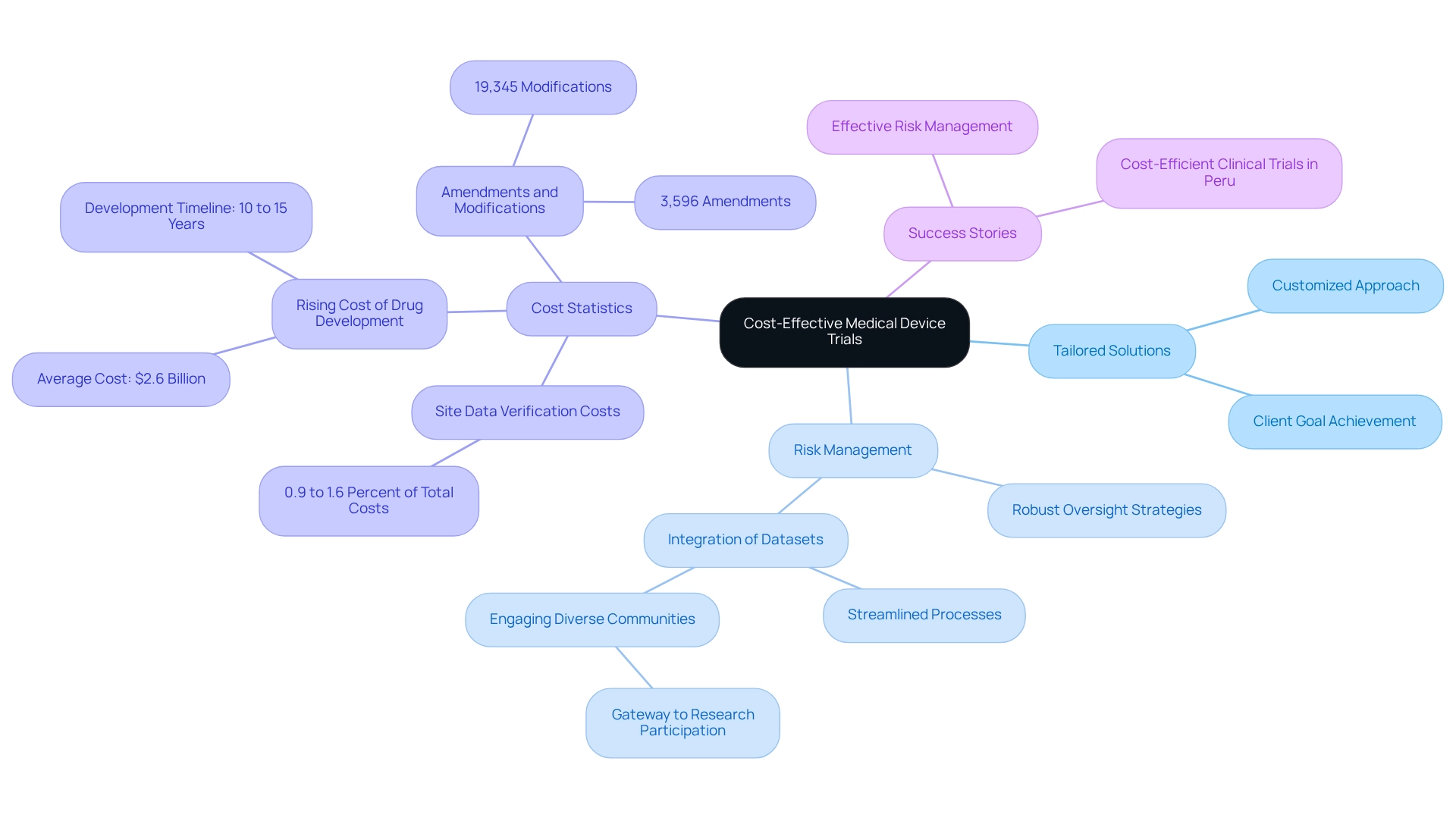
Huma: Digital-First Solutions for Cost-Efficient Clinical Trials
Huma leverages digital technology to create patient-focused trials that significantly enhance engagement and retention. Their innovative platform enables remote monitoring and data collection, effectively reducing the need for in-person visits and the associated costs. By adopting a digital-first strategy, Huma not only improves the patient experience but also substantially decreases operational expenses. This approach is in line with the growing trend in the research industry, where:
- 85% of stakeholders recognize the critical importance of technology adoption for maintaining competitiveness.
- Case studies reveal that while 48% of research sites express concerns about affording new technology, an impressive 83% of sponsors are prepared to provide electronic Investigator Site Files (eISFs) to sites lacking them.
This willingness to support technology implementation is essential for ensuring compliance with research regulations and fostering a more efficient research environment. In Latin America, comprehensive study management services, such as those offered by bioaccess™, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
are essential for facilitating cost-efficient clinical trials in Peru. The collaboration between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health, aims to position Barranquilla as a key hub for research studies, contributing to local economic development and job creation. As the sector evolves towards standardization, it is vital to establish shared definitions regarding digital solutions in medical studies, which will inform future reporting and enhance the overall effectiveness of health research in Latin America.
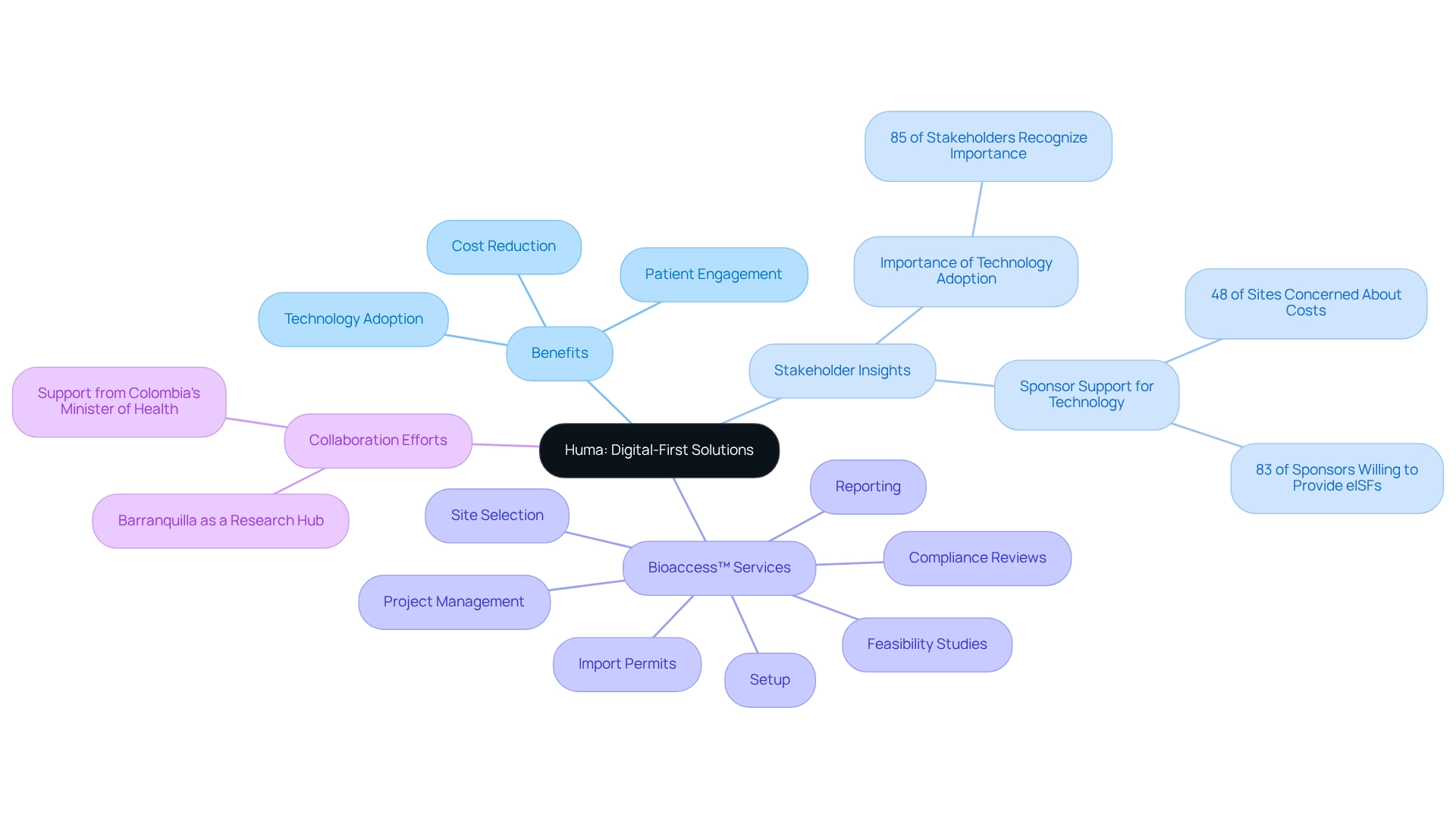
Linical: Streamlining Data Management for Cost-Effective Trials
Bioaccess offers a comprehensive suite of clinical study oversight services designed to significantly streamline feasibility assessments, site selection, compliance evaluations, study setup, import permits, project coordination, and reporting processes. These meticulously crafted solutions enhance data integrity while effectively reducing testing-related expenses. By implementing robust oversight techniques, bioaccess empowers clients to not only shorten testing timelines but also lower overall costs. This approach is particularly crucial in an era of rising medical research expenses, as cost-efficient clinical trials in Peru can lead to substantial savings.
Organizations that embrace effective oversight strategies often witness a marked decrease in operational costs, which allows for the allocation of more resources towards cost-efficient clinical trials in Peru. Furthermore, bioaccess conducts thorough reviews and provides feedback on study documents to ensure compliance with national requirements, facilitates the import permit process, and delivers detailed reporting on study status, inventory, and adverse events.
The healthcare research services sector is increasingly vital as it fosters global health advancements through international collaboration and innovation in medtech. Expert insights indicate that the integration of advanced oversight technologies can accelerate testing processes, ensuring that innovative medical devices reach the market swiftly and effectively.
Moreover, the latest trends in remote and virtual medical studies underscore the necessity for strong management solutions, further enhancing research effectiveness. As the landscape of clinical research evolves, the importance of collaboration and strategic oversight becomes paramount, guiding organizations toward improved outcomes and greater efficiency.
CureMatch: Enhancing Therapy Development for Cost-Effective Clinical Trials
CureMatch significantly enhances therapy development by delivering actionable insights that inform study design and execution. Their innovative platform meticulously analyzes patient data to pinpoint the most promising treatment options, facilitating more efficient study designs that ultimately reduce expenses. By prioritizing efficient therapy advancement, CureMatch streamlines the research process, leading to substantial savings in both time and resources. This approach not only boosts the overall efficiency of research studies but also aligns with the growing demand for cost-efficient clinical trials in Peru, especially in the Medtech sector, where the success rates for transitioning from Phase 2 to Phase 3 studies stand at 58.3%, and from Phase 3 to approval at 59.0%.
As Andrew W. Lo underscores, refining research design is crucial for maximizing returns on investment within the Medtech landscape. Furthermore, the global market for AI in medical studies is projected to grow at a CAGR of 22.1% from 2023 to 2028, highlighting the increasing importance of platforms like CureMatch.
In Latin America, companies such as bioaccess® provide comprehensive clinical research management services, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Post-Market Clinical Follow-Up Studies
These services contribute to local economies through job creation and advancements in healthcare. Insights from the case study titled 'Comparative Analysis of Drug Development Programs' reveal that varying success rates across therapeutic areas necessitate cost-efficient clinical trials in Peru to ensure that investments yield the highest possible returns.
Conclusion
The clinical trial landscape is rapidly evolving, particularly in Latin America, driven by the need for cost-effective and efficient solutions. Organizations like bioaccess® are at the forefront, offering tailored services that streamline trial processes while ensuring compliance with regulatory standards. This approach not only accelerates medical device development but also enhances patient outcomes.
Innovative strategies such as intelligent patient matching, virtual trials, and comprehensive regulatory consulting are essential for optimizing clinical trial management. Companies like CureMatch and Novotech demonstrate how technology can improve recruitment efficiency and reduce operational costs, encouraging wider participation in research. Collaborative efforts to establish regions like Barranquilla as key clinical trial destinations further enhance this transformation.
As the demand for affordable clinical trials increases, embracing digital solutions and effective data management becomes crucial. Medtech companies that prioritize these innovations can navigate the complexities of clinical research while lowering costs. Upholding ethical practices and regulatory excellence is vital for ensuring successful trials and improving healthcare systems.
In conclusion, the ongoing changes in clinical trials present organizations with an opportunity to rethink their strategies. By leveraging the expertise of companies like bioaccess®, stakeholders can achieve significant cost savings and operational efficiencies, ultimately leading to better health outcomes for patients. The future of clinical research lies in fostering a more effective and patient-centric approach to medical advancements.
Frequently Asked Questions
What services does bioaccess® provide for medical device development in Peru?
bioaccess® offers tailored research services that expedite medical device development, including early feasibility studies, first-in-human studies, pilot studies, and critical evaluations.
How does bioaccess® help reduce the costs of clinical trials in Peru?
bioaccess® leverages its knowledge of local regulatory systems and operational efficiencies to conduct cost-efficient clinical trials, with strategies such as optimizing site selection and enhancing patient recruitment.
What advantages do clinical trials in Peru have compared to other regions?
Clinical trials in Peru have significantly lower average expenses compared to many other regions, making it an attractive option for Medtech firms.
What is the significance of compliance with standards like FDA or CE in the research process?
Compliance requires rigorous testing and documentation, which can be time and resource-intensive, but it ensures adherence to stringent standards and ultimately leads to improved patient outcomes.
What comprehensive research study management services does bioaccess® offer?
bioaccess® provides services such as feasibility assessments, compliance evaluations, study setup, import permits, project oversight, and reporting.
How can Medtech companies maximize cost efficiency in their research?
Companies can implement strategies such as conducting cost-efficient clinical trials in Peru and continuously evaluating their testing processes for potential enhancements.
What role does CureMatch play in patient recruitment for clinical studies?
CureMatch uses advanced algorithms to connect patients with clinical studies tailored to their genetic profiles, which accelerates recruitment and enhances the likelihood of success.
How does the partnership between bioaccess™ and Caribbean Health Group aim to improve research in Latin America?
The partnership aims to position Barranquilla as a premier hub for research studies, enhancing patient recruitment and stimulating local economies through job creation and healthcare improvements.
What are Virtual Clinical Trials (VCTs) and their benefits?
VCTs enable remote patient observation and data collection, eliminating the need for physical testing locations, which reduces costs associated with site administration and patient transportation by up to 30%.
How do electronic patient-reported outcomes (ePROs) enhance research quality?
The integration of ePROs enhances data accuracy, improving the overall quality of research findings.
What are some of the study management services provided by bioaccess® related to VCTs?
bioaccess® offers services including early-feasibility studies, first-in-human studies, and post-market follow-up studies.




