Introduction
In the complex landscape of clinical research, the importance of meticulously crafted budgets cannot be overstated. These financial frameworks serve as the backbone for successful trials, delineating anticipated costs and guiding resource allocation.
As clinical trials grow increasingly intricate, understanding the nuances of budgeting—including the differentiation between direct and indirect costs—becomes essential for stakeholders aiming to navigate this demanding environment.
With the potential for significant economic impacts on local communities and healthcare advancements, the ability to develop, negotiate, and manage clinical trial budgets effectively is paramount.
This article delves into the critical components of clinical trial budgeting, offering insights into best practices and strategies that enhance trial feasibility and financial sustainability.
Understanding Clinical Trial Budgets: Key Concepts and Importance
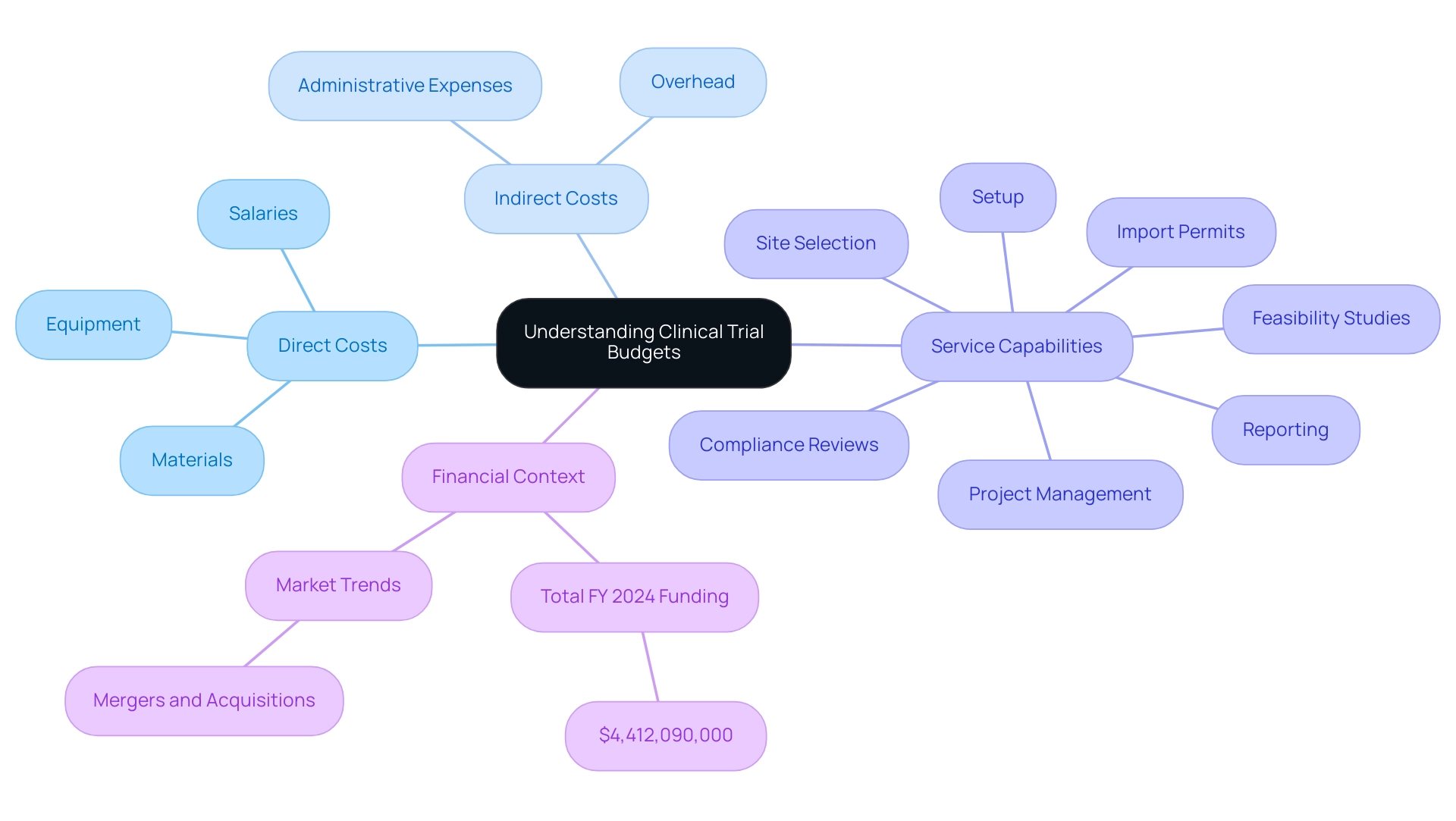
A clinical trial budget example highlights that clinical study financial plans are complex documents encompassing all expected expenses related to conducting a study. These budgets are typically divided into two primary categories:
-
Direct costs
- Salaries
- Equipment
- Materials
-
Indirect costs
- Overhead
- Administrative expenses
A thorough understanding of these components is crucial, as they significantly impact the project's feasibility, timeline, and resource allocation.
In the realm of clinical study management, our service capabilities enhance this process, offering:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Our expertise in Regulatory Affairs ensures that all aspects of the study comply with necessary regulations, while our insights into commercialization strategies help stakeholders navigate the complexities of bringing a product to market. A meticulously structured clinical trial budget example serves as a strategic roadmap for financial planning, ensuring adherence to regulatory and ethical standards.
Ultimately, this contributes to the successful execution of the experiment, facilitating smoother operations and better resource management. As the landscape of medical research continues to evolve—especially with the growing recognition of the economic impact of medtech studies on local economies, such as job creation and healthcare improvement—stakeholders must prioritize effective budgeting to navigate these complexities. Recent trends and funding projections, including the total FY 2024 funding amount of $4,412,090,000, underscore the need for informed financial strategies.
As mentioned by Samruddhi Yardi, a Market Research Analyst, comprehending the dynamics of research costs is crucial for making informed choices in this constantly evolving environment.
Steps to Develop an Effective Clinical Trial Budget
Creating a robust clinical trial budget is essential for the success of any research initiative and involves a systematic approach with several key steps:
- Define the Scope of the Study: It is crucial to comprehensively outline the study's objectives, methodologies, and timelines. This clarity enables a more accurate estimation of the resources required for a clinical trial budget example, ensuring that all facets of the trial are appropriately funded. Furthermore, feasibility studies and site selection, including the identification of a qualified principal investigator (PI), play a vital role in this phase, maximizing the potential for successful outcomes.
- Identify Direct and Indirect Expenses: A thorough categorization of expenses is vital. Direct expenses include outlays such as participant recruitment and site fees, while indirect expenses encompass overhead and administrative charges. This distinction ensures that the clinical trial budget example adequately covers all potential financial obligations. Significantly, start-up expenses may encompass IRB preparation, regulatory document preparation, FDA audit fees, and administrative costs, usually under $5,000 per site, but can differ based on study type and location.
- Consult with Stakeholders: Engaging with key stakeholders—including team members, sponsors, and regulatory bodies—provides invaluable insights and helps align expectations. Such collaboration is a cornerstone of effective budgeting, as it fosters a shared understanding of financial constraints and objectives. This is also vital for compliance assessments and setup processes, ensuring all necessary approvals are secured and that study documents are reviewed and feedback is provided in accordance with country requirements.
- Use Historical Data: Utilizing previous clinical trial budget examples can serve as a benchmark for current projections. Historical data not only aids in avoiding common budgeting pitfalls but also enhances the accuracy of cost estimations based on previous experiences. This practice can significantly impact the efficiency and success of trial management.
- Review and Revise: It is imperative to conduct regular assessments of the financial plan against actual expenditures. Continuous monitoring permits prompt modifications, allowing the finances to stay in sync with changing project requirements and economic conditions. Frequent budget evaluations are crucial to pinpoint and remove inefficiencies, thereby preserving the return on investment (ROI) of research studies. As highlighted in the case study titled "Identifying and Eliminating Budget Inefficiencies," these practices are necessary to mitigate financial risks and enhance the success of research sites.
Rosana Felice, Chief Medical Officer in biotech, underscores the significance of these practices:
Thanks, Basia Coulter, Ph.D. for this extraordinary analysis of cost-reduction opportunities in development. These types of solutions will enable faster access to innovative medicines.
By incorporating these strategies, budgeting for research studies can evolve into a more efficient process that ultimately supports the swift delivery of new therapies.
A comprehensive approach that is both data- and patient-focused is crucial for enhancing research timelines, decreasing expenses, and promoting economic development through job creation and healthcare advancements in local communities.
Negotiating Budgets: Strategies for Success with Sponsors
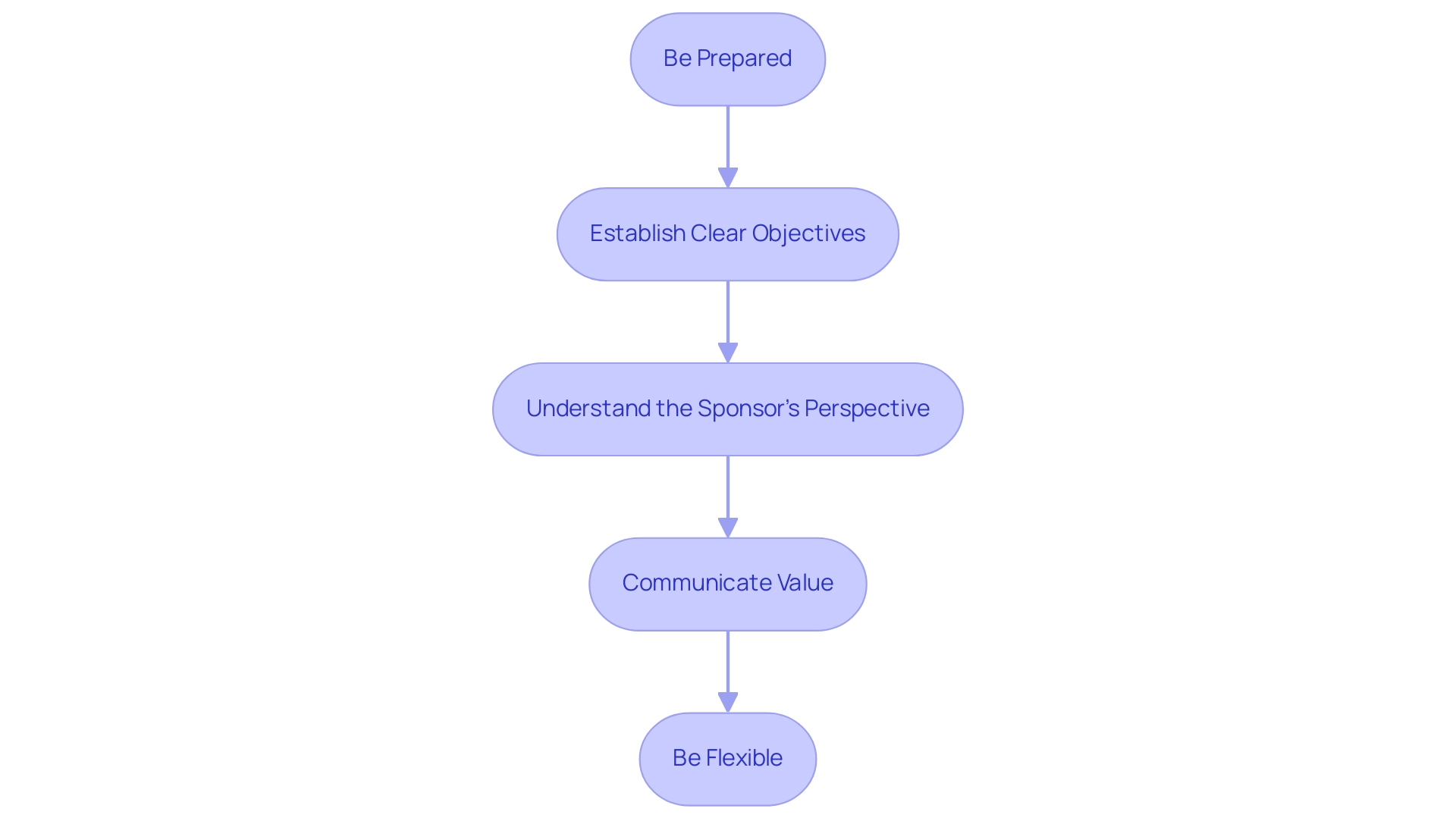
Negotiating financial plans with sponsors poses considerable difficulties, but it is essential for obtaining the required funding as illustrated in a clinical trial budget example for clinical studies. Here are effective strategies to enhance your negotiation outcomes:
-
Be Prepared: Gather all relevant data, including historical financial information and detailed cost estimates related to site payments, vendor expenses, and staffing costs.
This preparation lays the foundation for a compelling case that can significantly influence negotiations, as illustrated by a clinical trial budget example.
-
Establish Clear Objectives: Specify your minimum acceptable financial plan and determine the resources necessary for carrying out an effective test. Clarity in your objectives helps in articulating your needs during discussions, especially when considering a clinical trial budget example.
-
Understand the Sponsor's Perspective: Grasping the sponsor's priorities and constraints allows you to navigate negotiations more effectively. This understanding can facilitate the identification of common ground, ultimately leading to more productive conversations.
-
Communicate Value: Clearly articulate how the proposed budget, as illustrated in a clinical trial budget example, aligns with the project's objectives and the potential return on investment for the sponsor.
As Nitya Maddodi, PhD, MBA, Business Operations Services Manager, notes,
By maximizing ROI, research sites can ensure sustainable growth and success in their research endeavors.
This perspective is vital in showcasing the mutual benefits of the clinical trial budget example.
-
Be Flexible: Maintain an openness to discussions and adjustments.
Flexibility can often lead to collaborative solutions that benefit both parties, fostering a productive relationship with sponsors.
Additionally, consider the insights from a case study involving a top 10 global pharmaceutical company, which aimed to improve site payments as part of its strategy to become the sponsor of choice. This initiative resulted in dramatic improvements in site payments, showcasing the effectiveness of strategic negotiation.
Utilizing these strategies, along with internal planning, financial knowledge, and flexibility, not only enhances the likelihood of successful negotiations but also positions your practice for increased revenue and prestige, contributing to academic and scientific advancements.
Assessing Feasibility: A Critical Step in Budget Planning
Evaluating feasibility is a crucial element of a clinical trial budget example, significantly impacting resource distribution and overall expense estimation. The following key elements should be meticulously evaluated during feasibility assessments:
- Site Selection: Thoroughly evaluate the capabilities and resources of potential research locations to ensure they can adequately meet study requirements. A carefully chosen location, as shown by ReGelTec's successful Early Feasibility Study with HYDRAFIL™ for chronic low back pain in Colombia, plays a vital role in managing expenses and improving study efficiency.
- Patient Recruitment: Analyze the availability of eligible participants within the target demographic. For instance, GlobalCare Clinical Trials has partnered with bioaccess™ to enhance clinical trial ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and a 95% retention rate. Grasping the participant pool is crucial to prevent undervaluing recruitment expenses, which can result in budget overruns.
- Regulatory Considerations: Gain a comprehensive understanding of the regulatory landscape that may impact budgetary needs. This involves recognizing any extra compliance expenses that may emerge from differing regulations in various areas. Notably, familiarity with INVIMA, Colombia's National Food and Drug Surveillance Institute, is crucial for medical device oversight and classification as a Level 4 health authority by PAHO/WHO.
- Timeline Analysis: Assess the proposed timeline for the proceedings, as any potential delays can have significant cost implications. A realistic timeline helps mitigate unforeseen expenses and ensures improved financial management.
- Stakeholder Engagement: Engage stakeholders early in the feasibility process to gather insights and align expectations. This collaborative approach not only enhances budget accuracy but also fosters a greater commitment to the project's success.
As emphasized in current discussions, pilot studies aim to “field-test logistical aspects of the future study and to incorporate these aspects into the study design.” Interestingly, recent news indicates that pilot studies should not be used for estimating effect sizes or conducting exploratory analyses of efficacy, underscoring the need for a focused approach in feasibility assessments. Furthermore, the maximum length for the 95% confidence interval for the Pearson correlation coefficient with a sample size of 30 is 1.25, highlighting the importance of statistical considerations in feasibility assessments.
By utilizing real-world data (RWD), researchers can enhance study protocols, ultimately improving recruitment feasibility and data quality. For example, a case study named "Conducting Feasibility Studies Using Real-World Data" demonstrates how defining research questions, identifying potential RWD sources, and assessing data quality can improve recruitment feasibility, data collection, sample size estimation, and study design optimization. This thorough evaluation guarantees that all essential elements are considered, leading to a more efficient clinical trial budget example and successful research execution.
Additionally, bioaccess® offers a variety of research management services, including feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. Their expertise in these areas is crucial for navigating the complexities of clinical studies in Latin America. Understanding these services is essential for accurate budgeting and effective feasibility assessments.
Overcoming Budgeting Challenges in Clinical Trials
Clinical study budgeting presents a myriad of challenges, yet with proactive strategies, these obstacles can be effectively managed:
- Unexpected Costs: To address unforeseen expenses, it is prudent to incorporate a contingency fund that accommodates potential overruns, as unexpected costs can significantly impact overall project finances.
- Regulatory Changes: Remaining vigilant about evolving regulations is essential. Budget requirements can shift, necessitating adjustments to ensure compliance while maintaining financial targets. Understanding the role of INVIMA, Colombia's national regulatory authority, is crucial, as it oversees medical device classification, provides review and feedback on study documents, and ensures adherence to local regulations.
- Resource Allocation: Efficient resource allocation is crucial. Regular monitoring of expenditures allows for timely adjustments, ensuring that funds are utilized optimally throughout the trial. This includes strategic feasibility studies and careful selection of research sites and principal investigators.
- Communication Gaps: Open communication among all team members and stakeholders is vital. This transparency helps address concerns and aligns expectations surrounding financial management, fostering a collaborative environment.
- Data Management: Establishing strong data management systems helps in precisely monitoring expenses and offering real-time insights into financial performance. These systems enhance decision-making capabilities, especially in light of the complexities highlighted by NU Sulzer, who notes that more complex and burdensome protocols are extending study cycle times, increasing costs and challenging patient recruitment and retention.
- Common Financial Management Challenges: Statistics indicate that the overall power of the ABIS strategy may fall below the target due to adjustments based on interim results, impacting financial forecasts. This highlights the significance of consistently aligning financial strategies with experimental progress to reduce financial risks.
- Case Study Insights: A retrospective analysis by TREAD Research, focusing on payment processes at Tygerberg Hospital, revealed significant insights into average payment per patient per visit and the timelines associated with payments from CROss and pharmaceutical companies. Such findings contribute to a deeper understanding of the financial dynamics in clinical studies, highlighting the importance of timely and precise budgeting practices.
- Strategic Management of Costs: In light of specific study designs, such as two-armed controlled experiments that require a significant treatment effect to bring a drug to market, managing budgets and sample sizes effectively becomes imperative. Notably, the maximum second stage sample size should not exceed twice the pre-planned second stage sample size, which is a critical consideration for budgeting in these studies. Proactive planning can reduce risks related to unexpected expenses, ensuring that the clinical trial budget example is used to adequately allocate resources to achieve project objectives. Additionally, our comprehensive clinical trial management services encompass all these aspects, from study project management to reporting on study status, ensuring a streamlined process that supports economic growth and healthcare improvements.
Conclusion
In the complex landscape of clinical trial budgeting, understanding the nuances between direct and indirect costs is essential for effective financial planning. A well-structured budget acts as a strategic roadmap, guiding resource allocation and ensuring compliance with regulatory requirements.
Key steps in budget development include:
- Defining the study's scope
- Categorizing costs
- Engaging stakeholders
- Utilizing historical data
- Maintaining flexibility through regular reviews
This systematic approach fosters collaboration and adaptability, aligning the budget with the trial's evolving needs.
Addressing challenges such as unexpected costs and regulatory shifts necessitates proactive strategies. Incorporating contingency funds, promoting open communication, and implementing robust data management systems can effectively mitigate financial risks and enhance trial success.
Ultimately, effective clinical trial budgeting transcends mere financial management; it is a strategic imperative that influences healthcare innovation. By prioritizing thorough budgeting, stakeholders can facilitate successful trial execution, contribute to local economic growth, and advance medical knowledge. Embracing these principles will enable stakeholders to navigate the complexities of clinical research, ensuring that valuable therapies reach those who need them most.
Frequently Asked Questions
What is a clinical trial budget?
A clinical trial budget is a complex financial plan that encompasses all expected expenses related to conducting a clinical study. It includes both direct and indirect costs.
What are the main categories of costs in a clinical trial budget?
The main categories are: 1. Direct costs, which include salaries, equipment, and materials. 2. Indirect costs, which include overhead and administrative expenses.
Why is understanding clinical trial budget components important?
Understanding these components is crucial as they significantly impact the project's feasibility, timeline, and resource allocation.
What services can enhance the clinical study management process?
Services that enhance clinical study management include feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting.
How does Regulatory Affairs contribute to clinical trial budgeting?
Regulatory Affairs ensures that all aspects of the study comply with necessary regulations, which is essential for maintaining adherence to regulatory and ethical standards.
What is the significance of effective budgeting in clinical trials?
Effective budgeting is vital for navigating the complexities of clinical trials and contributes to the successful execution of the experiment, facilitating smoother operations and better resource management.
What are the steps involved in creating a robust clinical trial budget?
The steps include: 1. Defining the scope of the study. 2. Identifying direct and indirect expenses. 3. Consulting with stakeholders. 4. Using historical data for benchmarks. 5. Reviewing and revising the budget regularly.
Why is stakeholder consultation important in the budgeting process?
Engaging with stakeholders provides invaluable insights, aligns expectations, and ensures compliance, which is essential for effective budgeting and securing necessary approvals.
How can historical data aid in clinical trial budgeting?
Historical data serves as a benchmark for current projections, helps avoid common budgeting pitfalls, and enhances the accuracy of cost estimations based on previous experiences.
What is the importance of reviewing and revising the clinical trial budget?
Regular assessments against actual expenditures allow for prompt modifications, helping to identify and remove inefficiencies, which preserves the return on investment (ROI) of research studies.




