Introduction
The pathways to securing FDA clearance for medical devices are critical for manufacturers aiming to bring innovative products to market. Among these pathways, the 510(k) and De Novo submissions stand out, each serving unique purposes in the regulatory landscape.
- The 510(k) submission, often favored for its expedited review process, allows manufacturers to demonstrate substantial equivalence to existing devices, facilitating quicker market access.
- Conversely, the De Novo pathway is tailored for novel devices that lack a predicate, requiring comprehensive evidence of safety and effectiveness.
As the medical technology sector continues to evolve, understanding these submission processes is essential for navigating the complexities of regulatory compliance and ensuring the successful introduction of groundbreaking healthcare solutions.
This article delves into the nuances of both pathways, exploring their key differences, advantages and disadvantages, and the regulatory considerations that can influence manufacturers' strategic decisions.
Understanding 510(k) and De Novo Submissions: An Overview
The 510 k de novo applications represent two essential routes for obtaining FDA approval for medical equipment, each serving unique functions. A 510(k) submission, also known as a premarket notification, is intended to show that a product is substantially equivalent to an existing marketed item, referred to as a predicate. This route is typically more efficient, making it the favored choice for items that closely resemble current products.
In fact, among the approved Breakthrough Products, there are 124 CDRH items and 4 CBER items, emphasizing the importance of the 510(k) route in enabling prompt access to innovative solutions. In contrast, the 510 k de novo submission process is specifically designed for innovative devices that lack a predicate, necessitating a risk-based classification approach. This route enables manufacturers to provide comprehensive evidence of safety and effectiveness for novel technologies.
By facilitating the introduction of groundbreaking medical solutions, the pathway of 510 k de novo plays a vital role in advancing healthcare while prioritizing patient safety. As the FDA notes, 'The FDA believes use of these best practices outlined in this draft guidance, once finalized, will encourage the evolution of safer and more effective medical products in the 510 k de novo process over time.' This demonstrates the agency's dedication to ongoing enhancement in the approval procedures for medical products.
Additionally, tools like Qualio's software can assist manufacturers in streamlining their FDA 510 k de novo application process, making compliance more manageable. Furthermore, four groups are responsible for submitting a 510(k):
- American medical product manufacturers
- Representatives of non-U.S. manufacturers
- Specification developers
- Repackers/relabellers
Each group has distinct responsibilities according to their position in the production and promotion of medical devices, such as ensuring that the device meets the required compliance standards and supplying sufficient documentation for the application.
In the context of Colombia, comprehending the legal framework set by INVIMA as a Level 4 health authority by PAHO/WHO is essential for managing both local and international applications. With experts like Ana Criado, who leads Regulatory Affairs and has extensive experience in compliance and project management, organizations can better align their clinical trial activities with regulatory expectations. Our comprehensive clinical trial management services encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting, all of which are vital for successful 510 k de novo approvals.
This thorough comprehension of the application routes and the related responsibilities is essential for effective navigation of the FDA approval landscape, along with adherence to Colombian regulations.
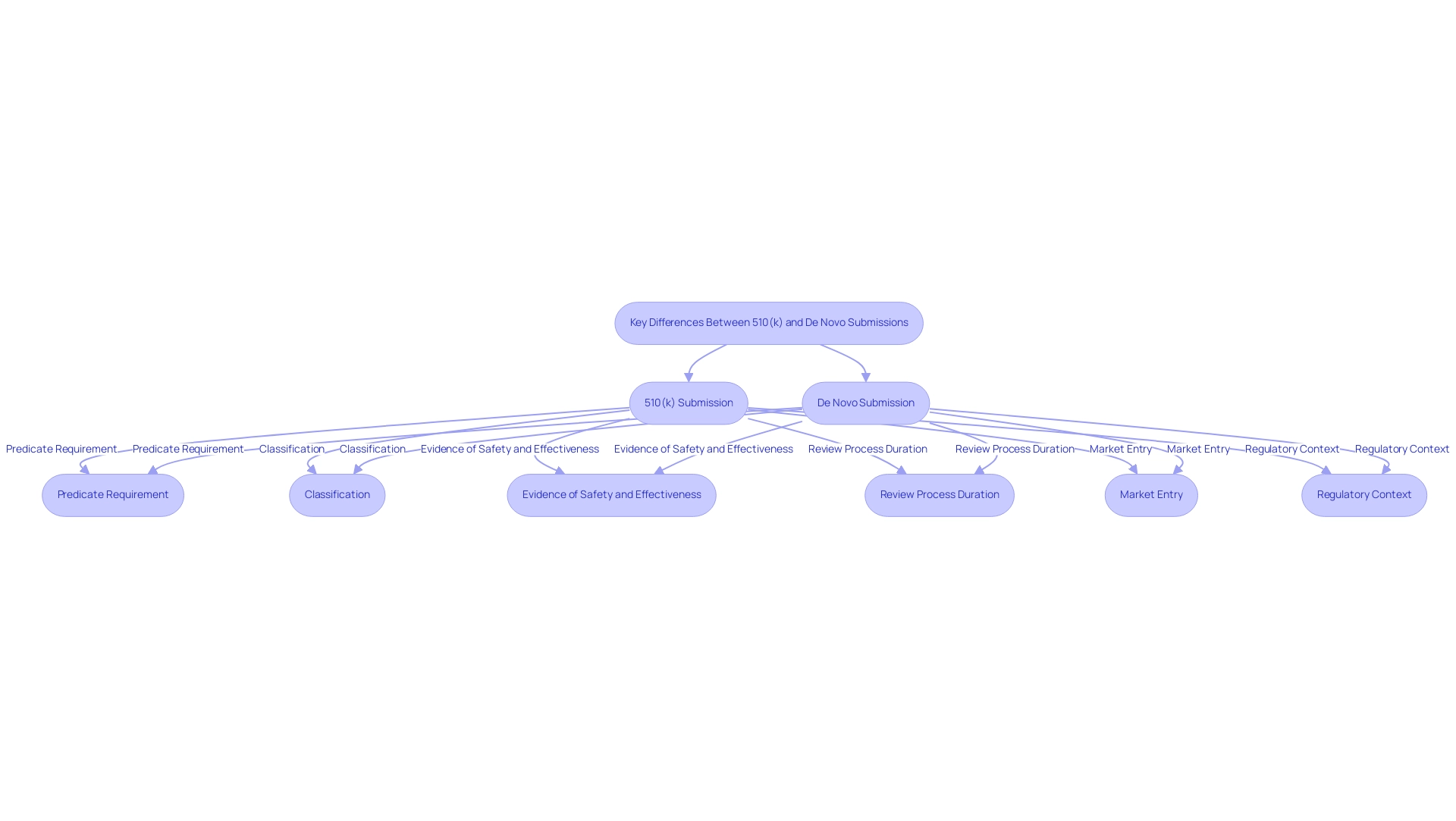
Key Differences Between 510(k) and De Novo Submissions
-
Predicate Requirement: The 510 k de novo submission process necessitates identifying a predicate product for comparison, which serves as a benchmark to demonstrate substantial equivalence. In contrast, the De Novo pathway is designed for products that lack a predicate, allowing for the introduction of innovative items that do not fit into existing categories.
-
Classification: Typically, items that undergo the 510(k) process are classified as Class II, reflecting their moderate risk. Conversely, De Novo applications are versatile, as they can be classified as either Class I or II depending on the associated risks, thus providing flexibility in regulatory categorization.
-
Evidence of Safety and Effectiveness: A fundamental distinction lies in the evidence required for approval. The 510 k de novo process highlights the need to prove substantial equivalence to a current device, while De Novo applications must present thorough data to demonstrate the safety and efficacy of new devices, frequently requiring more extensive clinical evidence.
-
Review Process Duration: The timeline for review is another critical difference. The 510(k) route generally facilitates a quicker review process, typically lasting around 90 days, with a letter communicating the FDA's decision usually received within 60 calendar days of the FDA receiving the request. In contrast, De Novo applications may experience prolonged evaluation periods due to their complexity and the necessity for thorough scrutiny of new data.
-
Market Entry: Devices that gain clearance through the 510 k de novo pathway can often enter the market more swiftly, capitalizing on their established equivalence to predicate devices. An example of this is the INSIGHTEC EXABLATE, which received marketing authorization (number P150038) on 07/11/2016, illustrating the practical implications of the 510 k de novo process. Conversely, De Novo submissions may involve additional steps prior to market access, reflecting the need for a more detailed assessment of their novel characteristics.
-
Regulatory Context: In Colombia, the INVIMA plays a similar role to the FDA in the U.S., overseeing the approval processes for medical equipment. Established in 1992, INVIMA is responsible for ensuring the safety, efficacy, and quality of health products, including medical devices. It functions under the Ministry of Health and Social Protection and is acknowledged as a Level 4 health authority by PAHO/WHO, indicating its proficiency in oversight duties. Comprehending INVIMA's framework is crucial for traversing compliance routes in Colombia, especially for businesses aiming to launch innovative medical technologies. Unlike the FDA's processes, INVIMA may have different timelines and requirements for device approval, which can impact market entry strategies.
-
Expert Insight: According to Jemin Dedania, Director of Regulatory Affairs at Hogan Lovells US LLP, "Understanding the distinctions between the 510 k de novo and 510(k) routes is crucial for effectively navigating the FDA's oversight framework, especially for companies seeking to introduce innovative medical technologies." This expert perspective further underscores the importance of recognizing the differences in these submission processes.
-
Conclusion: For Clinical Research Directors, comprehending both the FDA and INVIMA processes is vital for effective planning and execution of clinical trials and product launches. The variations in oversight routes can greatly affect schedules and market approaches for medical equipment.
Pros and Cons of 510(k) vs. De Novo Submissions
510(k) Submission:
Pros:
- Offers faster market access due to shorter review timelines, typically ranging from 3 to 6 months.
- Provides an established pathway supported by a wealth of precedent, thereby reducing uncertainty in the approval process.
- Typically involves a reduced regulatory burden for products that can demonstrate substantial equivalence to existing offerings, particularly for those utilizing the 510(k) de novo process, making it a favored option among manufacturers.
Cons:
- Limited to devices that can demonstrate substantial equivalence to a predicate, which can restrict innovation in certain cases.
- May face significant challenges if no predicate item exists, leading to an extended approval timeline. For instance, Fifth Eye experienced an 11-month FDA review process, which included at least five major interactions with the agency, emphasizing the potential complexities even within this established pathway.
De Novo Submission:
Pros:
- Facilitates the introduction of innovative devices that lack a predicate, paving the way for novel technologies to enter the market.
- Holds the potential for a Class I designation, which may substantially reduce oversight requirements and expedite market entry.
Cons:
- Involves a longer review process, often extending beyond a year, along with more extensive data requirements to satisfy regulatory scrutiny.
Fifth Eye took six months to prepare their De Novo submission, illustrating the time investment required even before review begins.
- Carries uncertainty regarding the regulatory pathway for new technologies, which can deter some manufacturers from pursuing this option. As noted by industry experts, the complexity of securing the FDA’s authorization creates both a competitive barrier and a significant value for those who can successfully navigate the process.
This competitive barrier is crucial in understanding the landscape of medical equipment approvals, particularly in relation to 510(k) de novo. Furthermore, challenges in FDA staffing, such as the high living costs affecting talent acquisition, can impact the thoroughness of reviews and contribute to the complexities faced by manufacturers.
INVIMA: Overview and Regulatory Functions
As a counterpart to the FDA in the United States, the INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) plays a critical role in Colombia’s health product regulations. Established in 1992 and classified as a Level 4 health authority by PAHO/WHO, INVIMA oversees the marketing and manufacturing of health products, ensuring compliance with safety and efficacy standards.
Additionally, INVIMA is responsible for the import and export of health products, which is crucial for maintaining the integrity of Colombia's health product market. The Directorate for Medical Equipment and Other Technologies within INVIMA is responsible for monitoring medical equipment and suggesting technical standards for their regulation. This framework is crucial for comprehending the Colombian oversight environment for medical equipment.
Expertise in [Regulatory Affairs
Ana Criado](https://fdli.org/2024/10/an-introduction-to-the-medical-device-approval-process-premarket-notification-510k-and-de-novo-requests), the Director of Regulatory Affairs, brings invaluable expertise in this field, having served in various leadership roles at INVIMA for over five years. Currently, she is a professor at prestigious institutions such as Universidad Javeriana and Universidad de los Andes and consults for major companies like General Electric and Omron Healthcare. Her extensive background in biomedical engineering, health economics, and cannabis regulation positions her as a leading figure in compliance matters.
Ana's knowledge is essential for maneuvering through the intricacies of medical equipment approvals in Colombia, especially in tackling compliance challenges that producers encounter in this changing environment.
Regulatory Considerations: Choosing Between 510(k) and De Novo
When navigating the choice between the 510 k de novo and traditional 510(k) routes, manufacturers must carefully evaluate several regulatory factors. The 510 k de novo application is generally preferred for products that can easily demonstrate substantial equivalence to current items, which can greatly speed up the approval process. In contrast, novel tools lacking a clear predicate may find the 510 k de novo pathway to be their only viable option.
For example, in the past year, two de novo requests were subjected to advisory panel review, including DEN150035 granted on February 3, 2017, highlighting the scrutiny these submissions may face. Moreover, a thorough understanding of the classification of the apparatus is essential, as it directly influences the regulatory requirements and the overall timeline for market entry. It is also important for manufacturers to be mindful of the differing post-market surveillance obligations that may apply to each route, as these can have significant implications for ongoing compliance and safety monitoring.
The analysis of De Novo requests from 1997 to August 2023 indicates that the majority of innovations are at the continuous improvement level rather than at the category level, emphasizing the need for strategic decision-making in this context. Furthermore, insights from the case study on the differences between the 513(g) and Pre-Submission processes offer guidance on selecting the suitable compliance route based on the specific requirements of the medical equipment company. Experts such as Ana Criado and Katherine Ruiz can offer valuable perspectives on navigating these compliance pathways, ensuring that manufacturers make informed decisions.
As emphasized by industry expert Mike Drues,
If you don't, then you run the risk of just becoming another one of those statistics,
highlighting the significance of making informed regulatory decisions in the competitive medical equipment landscape.
Navigating the Submission Process: Timelines and Requirements
- Preparation Phase: The initial step for manufacturers involves meticulously gathering all requisite documentation and data, a process that can span several months depending on the device's complexity. This phase is crucial, as incomplete entries can lead to delays in the approval process. Engaging in a pre-submission consultation with the FDA may add an additional 60 to 75 days to the overall timeline but can significantly clarify expectations and requirements. Furthermore, leveraging comprehensive clinical trial management services, such as feasibility studies, compliance reviews, trial setup, import permits, and monitoring, can enhance preparation quality. Utilizing Qualio's software can also help streamline the FDA application process for 510 k de novo, making it more efficient by providing tools for documentation management and compliance tracking. Katherine Ruiz, an expert in Regulatory Affairs for medical products and in vitro diagnostics in Colombia, emphasizes the importance of thorough preparation to ensure compliance.
- Submission Timeline: After sending, the FDA aims to review 510(k) applications within an average of 90 days. In contrast, applications for 510 k de novo may extend to 150 days or longer, reflecting the increased complexity often associated with novel devices. For instance, the longest documented review took 287 days for BrainLab’s BOLD MRI Mapping tool, with Dr. Hugh Harvey noting that such variability underscores the importance of strategic planning. This highlights not only the potential delays but also the need for manufacturers to prepare adequately.
- Post-Submission Activities: After presenting their materials, manufacturers should stay alert and ready for possible inquiries for extra information from the FDA. Such inquiries can significantly extend the review timeline, emphasizing the need for thorough initial entries. Proactive communication and timely responses can mitigate these delays, ensuring a smoother review process. It is essential for manufacturers to be aware of the challenges currently facing the 510 k de novo process, including review volume and predicate product creep, which have prompted calls for revitalization in the approval process.
- Final Decision: Upon completing its review, the FDA issues a decision letter to the manufacturer, detailing the outcome of the submission. This letter not only confirms the approval or denial of the device but also outlines any necessary follow-up actions or modifications required for compliance. Understanding this final step is essential for manufacturers to effectively navigate the post-approval landscape.
Conclusion
Navigating the regulatory pathways for medical device approval is a complex yet essential undertaking for manufacturers aiming to introduce innovative healthcare solutions. The 510(k) and De Novo submissions each offer distinct advantages and challenges, tailored to different types of devices.
-
The 510(k) pathway, with its focus on substantial equivalence, allows for expedited market access, benefiting manufacturers with devices closely resembling existing products. However, this route can be limiting for truly novel innovations that do not have a predicate device.
-
On the other hand, the De Novo pathway presents opportunities for groundbreaking technologies that lack direct comparisons, albeit with more extensive data requirements and longer review timelines.
The choice between these submission processes requires careful consideration of regulatory factors, including:
- Device classification
- Market entry strategies
- Post-market obligations
Ultimately, understanding these regulatory frameworks is crucial for manufacturers to make informed decisions that align with their product development strategies. Whether opting for the quicker 510(k) route or the more thorough De Novo pathway, the ability to navigate these processes effectively can significantly impact a device's success in the marketplace. As the medical technology landscape continues to evolve, a firm grasp of these pathways will empower manufacturers to bring innovative solutions to healthcare, ultimately enhancing patient outcomes and advancing the industry.
Frequently Asked Questions
What are the two essential routes for obtaining FDA approval for medical equipment?
The two essential routes are the 510(k) submission and the 510(k) de novo submission. The 510(k) submission shows that a product is substantially equivalent to an existing marketed item (predicate), while the de novo submission is for innovative devices that lack a predicate.
What is a 510(k) submission?
A 510(k) submission, also known as a premarket notification, is intended to demonstrate that a product is substantially equivalent to a predicate device. It is typically a more efficient route for items that closely resemble existing products.
What is the purpose of the 510(k) de novo submission process?
The 510(k) de novo submission process is designed for innovative devices that do not have a predicate. It requires a risk-based classification approach and allows manufacturers to provide comprehensive evidence of safety and effectiveness for new technologies.
How does the FDA support the 510(k) de novo process?
The FDA emphasizes the use of best practices in the 510(k) de novo process to encourage the development of safer and more effective medical products, demonstrating its commitment to improving approval procedures.
Who is responsible for submitting a 510(k)?
Four groups are responsible for submitting a 510(k): American medical product manufacturers, representatives of non-U.S. manufacturers, specification developers, and repackers/relabellers. Each group has specific responsibilities regarding compliance and documentation.
What is the significance of understanding INVIMA in Colombia?
Understanding INVIMA's legal framework is essential for managing local and international applications for medical devices, as it functions similarly to the FDA in overseeing the approval processes and ensuring product safety and efficacy.
What are the main differences between 510(k) and de novo submissions?
The main differences include: Predicate Requirement: 510(k) requires a predicate for comparison, while de novo does not. Classification: 510(k) items are typically Class II, while de novo can be Class I or II. Evidence of Safety and Effectiveness: 510(k) focuses on substantial equivalence, whereas de novo requires comprehensive data on safety and efficacy. Review Process Duration: 510(k) generally has a quicker review process (around 90 days), while de novo may take longer due to complexity.
How does the market entry process differ for devices approved through 510(k) and de novo pathways?
Devices cleared through the 510(k) pathway can often enter the market more quickly due to established equivalence to predicate devices. In contrast, de novo submissions may involve additional steps and a more detailed assessment before market access.
What role does expert insight play in understanding the FDA's approval processes?
Experts, such as Jemin Dedania, emphasize the importance of understanding the distinctions between the 510(k) and de novo routes for effectively navigating the FDA's oversight framework, especially for companies introducing innovative medical technologies.
Why is it important for Clinical Research Directors to understand both FDA and INVIMA processes?
Comprehending both processes is vital for effective planning and execution of clinical trials and product launches, as variations in oversight routes can significantly affect schedules and market strategies for medical equipment.




