Introduction
The landscape of medical device regulation is undergoing a profound evolution, particularly in the context of De Novo and 510(k) submissions. As manufacturers navigate these two distinct pathways, understanding their unique requirements and implications becomes paramount.
The 510(k) submission offers a streamlined approach for devices that can demonstrate substantial equivalence to existing products, while the De Novo pathway opens doors for innovation by accommodating novel devices without a predicate.
With industry leaders increasingly favoring U.S. regulatory approval, the stakes are high for manufacturers aiming for market entry.
This article delves into the intricacies of both submission types, exploring their processes, advantages, and challenges, and highlights the strategic considerations that manufacturers must weigh to ensure compliance and successful commercialization in a dynamic regulatory environment.
Understanding De Novo and 510(k) Submissions: Definitions and Key Concepts
The de novo 510 k application and the 510(k) application signify two crucial routes for obtaining FDA approval for medical instruments, each fulfilling different roles in the compliance environment. The 510(k) submission is utilized for products that can demonstrate substantial equivalence to an already marketed item, facilitating a more streamlined approval process. Conversely, the de novo 510 k pathway caters to novel devices lacking a predicate, enabling a more tailored and comprehensive assessment suited to their unique characteristics.
Industry leaders, including specialists like Ana Criado, Director of Compliance and CEO of Mahu Pharma, emphasize the significance of these pathways, particularly as 90 percent of manufacturers express a preference for U.S. approval over EU pathways. Ana's extensive experience at INVIMA, where she held various executive roles, along with her consultancy for global companies such as General Electric and Omron Healthcare, equips her with unique insights into the compliance complexities that manufacturers face. Understanding these definitions becomes vital for manufacturers, influencing their market entry strategies and ensuring compliance with FDA regulations.
Notably, for the year leading up to September 2022, a substantial 67 percent of FDA 510(k) applications prompted requests for additional information during the substantive review process, underscoring the importance of precise and thorough entries in navigating these pathways to achieve successful clearance. Furthermore, the recent withdrawal of accreditation for Accelerated Device Approval Services, LLC, finalized on 08/13/2021, highlights the changing compliance landscape that manufacturers must navigate. As pointed out by regulatory specialists, their contributions to the manuscript included writing sections related to SaMD products and interpreting results, emphasizing the complexities involved in these entries.
Additionally, the FDA's guidance on best practices for selecting a predicate product, focusing on established methods and safety performance, aims to promote the development of safer and more effective medical instruments within the 510(k) Program.
Key Differences Between De Novo and 510(k) Submissions: Requirements and Processes
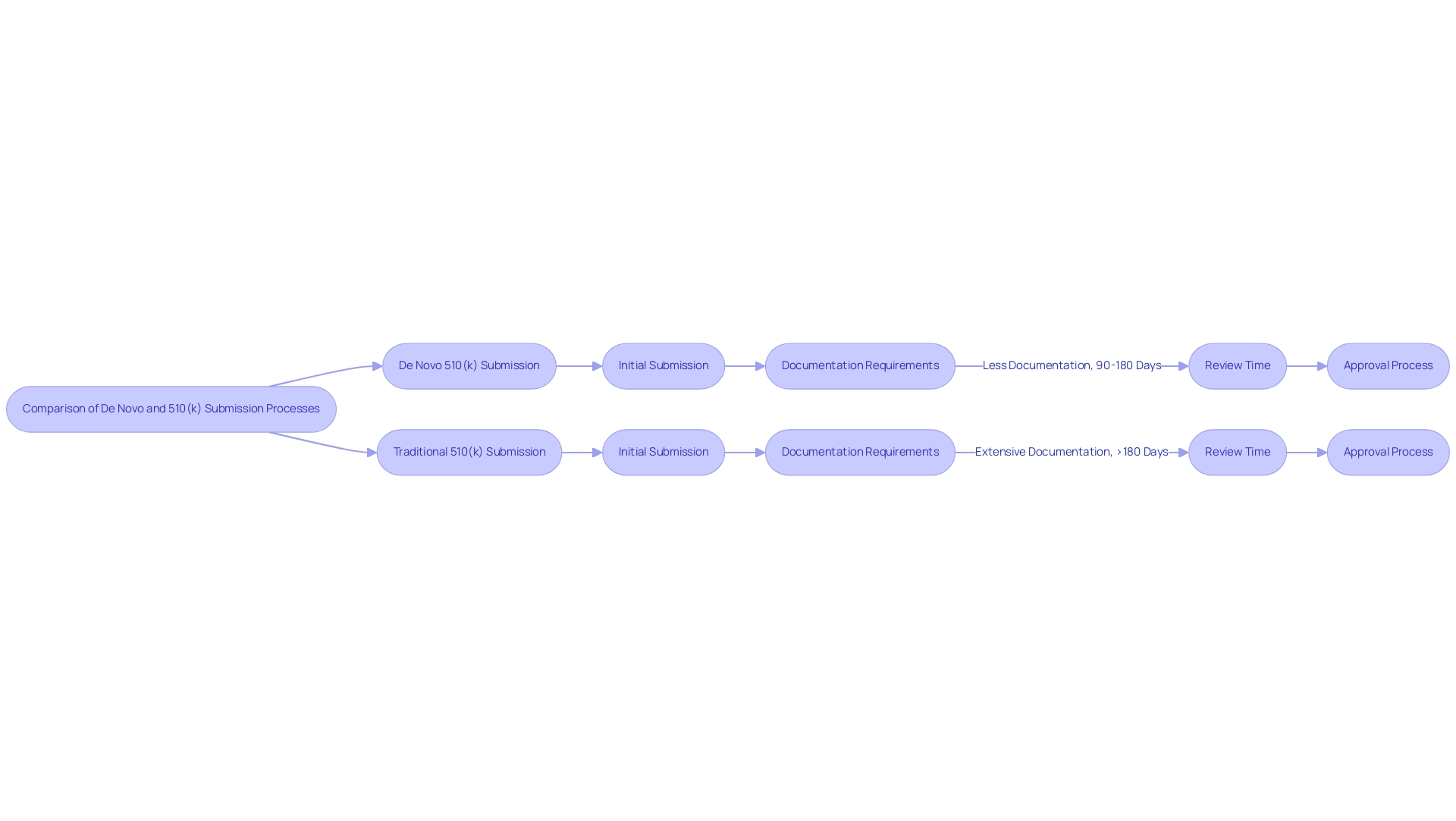
The application process for the de novo 510(k) is characterized by its relatively straightforward requirements, typically necessitating less documentation and review times that generally span from 90 to 180 days, with an average of 3.9 months. This process relies on showing substantial equivalence to an existing product, facilitating a more streamlined pathway for manufacturers. Once a product obtains 510(k) approval, it can serve as a reference for future applications, boosting its market appeal.
In contrast, the de novo 510(k) application process is inherently more complex, often requiring extensive documentation, including clinical data, to thoroughly evaluate the device's safety and effectiveness. As a result, timelines for de novo 510(k) applications can exceed 180 days, with recent statistics indicating a slight increase in approval wait times, rising from 415 days in 2023 to 420 days in 2024. Additionally, the average Panel Track wait times have also increased from 285.80 days to 289.62 days, underscoring the variability in compliance efficiency.
Manufacturers must meticulously assess their product's classification to ascertain the most suitable submission route, especially in light of these evolving timelines. As noted by Dr. Hugh Harvey, 'The slowest was BrainLab at 287 days for their BOLD MRI Mapping tool,' illustrating the potential for significant delays even within the 510(k) process. Furthermore, it's worth noting that CE marking takes an average of 12.1 months compared to 16.4 months for FDA 510(k) clearance, providing a broader context for the timelines discussed.
In Colombia, the monitoring framework is managed by INVIMA, the national food and drug oversight authority, which plays an essential role in the approval process for medical instruments. With specialists like Ana Criado, who is the Director of Compliance at Mahu Pharma, and Katherine Ruiz, an expert in Affairs for Medical Devices and In Vitro Diagnostics, the framework is strengthened by professionals with extensive backgrounds in biomedical engineering and health economics. They ensure that compliance and oversight are effectively managed throughout the clinical trial process, including services such as feasibility studies, trial setup, project management, and reporting on study status and adverse events.
Pros and Cons of De Novo vs. 510(k) Submissions: Evaluating Your Options
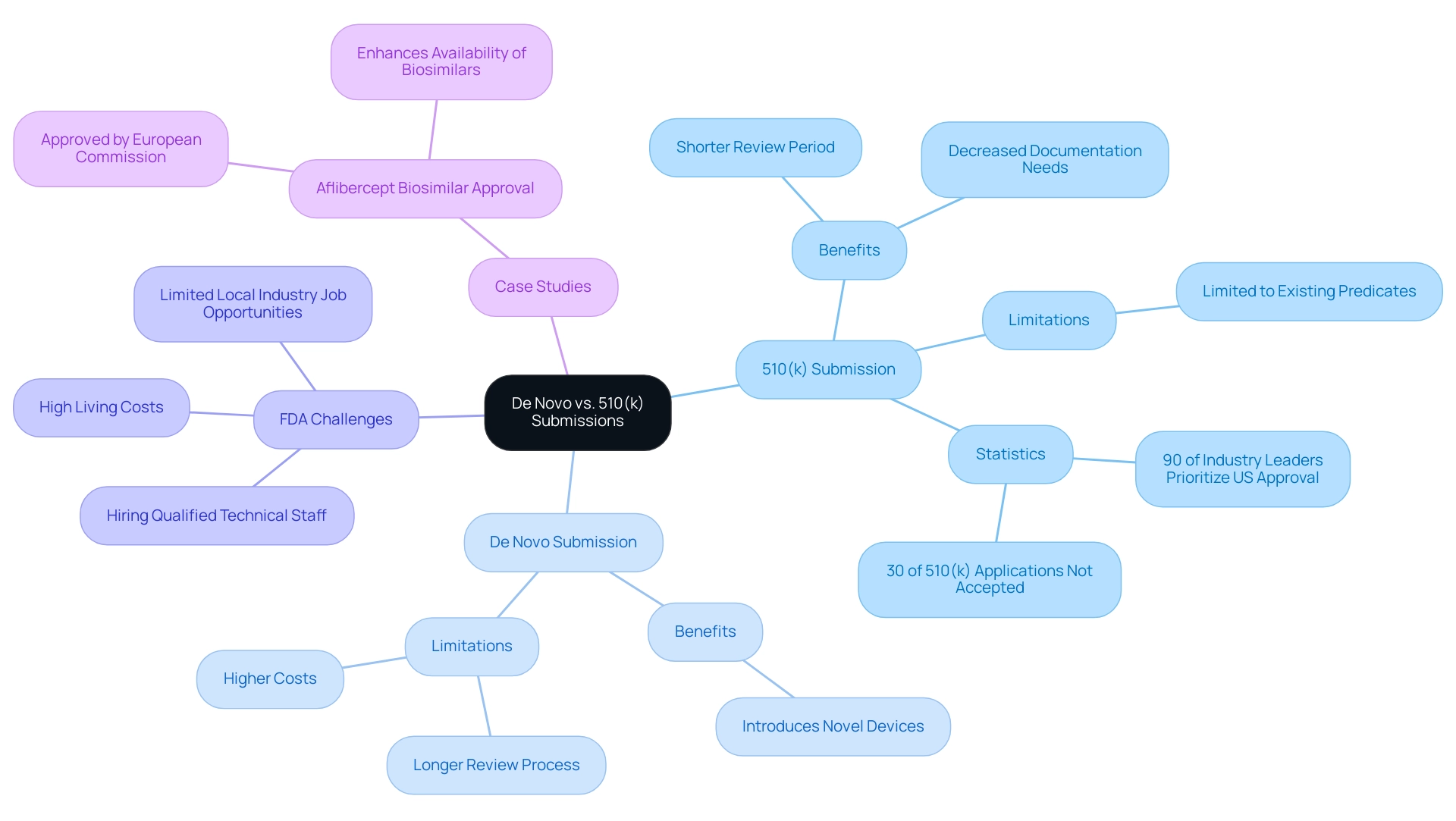
Assessing the 510(k) submission pathway uncovers substantial benefits, including a typically shorter review period and decreased documentation needs, rendering it an efficient option for established products. This is particularly relevant as 90 percent of industry leaders now prioritize US regulatory approval over the EU, reflecting the importance of the 510(k) pathway in the current regulatory landscape. However, this pathway is limited to items with an existing predicate, which may restrict innovation.
Conversely, the de novo 510(k) pathway presents an avenue for introducing novel devices into the market, yet it is accompanied by a longer review process and potentially higher costs, primarily due to the extensive clinical data required for approval. As highlighted by recent statistics, in 2022, 30% of 510(k) applications were not accepted for initial review, often due to common errors like incorrect templates and disorganized documentation. This underscores the importance of meticulous preparation for manufacturers.
Given our comprehensive clinical trial management services, including:
- feasibility studies
- trial set-up
- start-up and approval processes (ethics committee and health ministry)
- compliance reviews
- reporting (study status, inventory, serious and non-serious adverse events)
we ensure that your application process is streamlined and efficient. Furthermore, the FDA's difficulties in recruiting skilled technical personnel owing to elevated living expenses and restricted local industry job prospects may further complicate application processes and timelines. Tim Ulatowski, director of the Office of Compliance at CDRH, noted that while the FDA sometimes shifts from surveillance to action, the integration between these functions is not optimal.
Furthermore, the recent approval of the Aflibercept biosimilar by the European Commission illustrates the contrasting approval processes, highlighting the advantages and challenges of both the 510(k) and de novo 510(k) pathways. Therefore, manufacturers must carefully weigh these factors against their product's market strategy when selecting a suitable approach. For expert guidance, Katherine Ruiz focuses on compliance matters for medical instruments and in vitro diagnostics in Colombia, ensuring adherence and effective navigation through these complex processes.
Strategic Considerations: Choosing Between De Novo and 510(k) Submissions
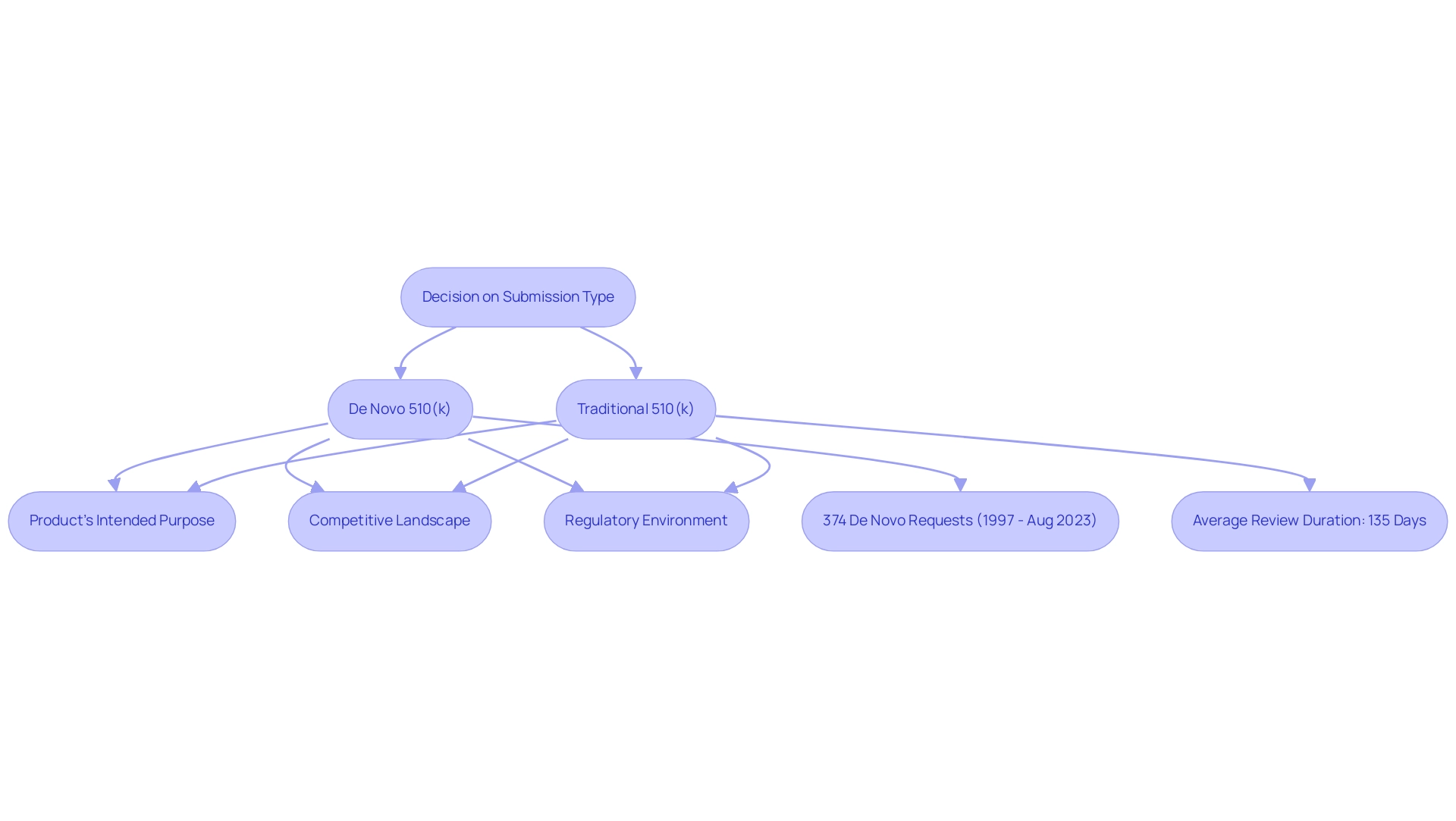
Selecting between applications for de novo 510(k) and traditional 510(k) necessitates a comprehensive evaluation of multiple essential elements, such as the product's intended purpose, the competitive landscape, and the regulatory environment. Experts like Ana Criado, our Director of Regulatory Affairs with a degree in chemical pharmacology and a master's in health economics & pharmacoeconomics, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, underline the importance of comprehensive knowledge in this area.
The de novo 510(k) pathway, which represents only about 0.89% of the size of the 510(k) pathway, has experienced a significant rise in requests, with a total of 374 de novo 510(k) classification requests submitted from 1997 to August 2023, averaging between 21 to 40 requests each year since 2017.
This trend indicates not only the expanding scope of de novo 510(k) submissions—historically limited to low-risk devices—to now include higher-risk technologies such as neurovascular mechanical thrombectomy devices and advanced ultrasound systems for tissue ablation, but also highlights the complexities in FDA reviews that may arise from advisory panel evaluations. Moreover, the average review duration for Radiology 510(k)s is approximately 135 days, which manufacturers should consider when assessing their strategies for proposals. Manufacturers should weigh these elements against their long-term goals, including market access timelines and potential barriers to entry.
By formulating a well-thought-out strategic plan, companies can navigate the compliance landscape more effectively, enhancing their prospects for successful product commercialization.
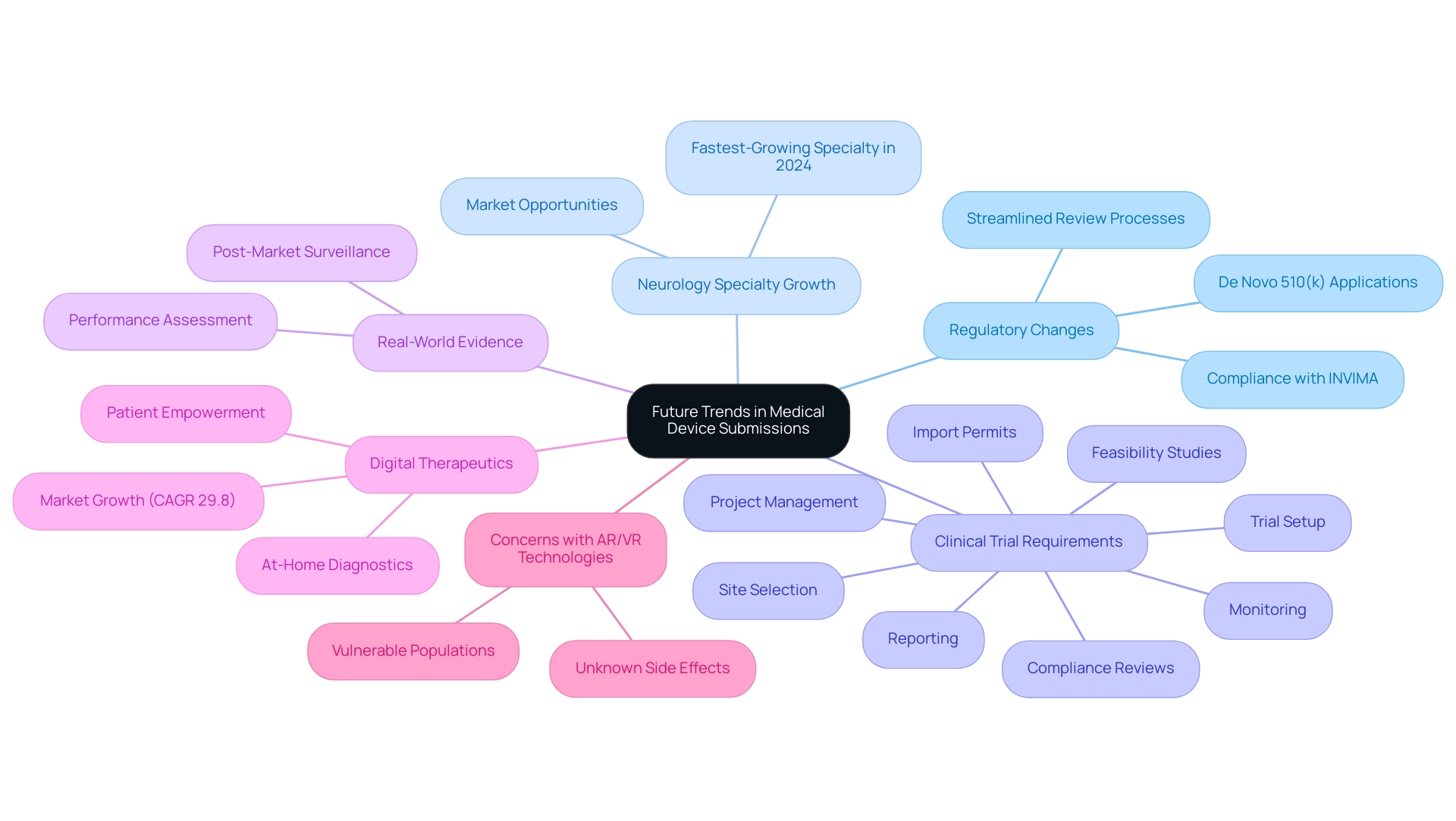
Future Trends in Medical Device Submissions: What to Expect for De Novo and 510(k)
The medical technology landscape is on the verge of substantial change, particularly regarding the regulatory structures related to de novo 510 k applications. As neurology emerges as the fastest-growing specialty in 2024, manufacturers must adapt to evolving requirements, including comprehensive clinical trial management services such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Monitoring
- Reporting
A pivotal shift towards incorporating real-world evidence and robust post-market surveillance is likely to reshape submission protocols, enhancing the FDA's ability to assess performance in real-world settings.
Furthermore, the oversight provided by INVIMA, Colombia’s National Food and Drug Surveillance Institute, as a Level 4 health authority certified by PAHO/WHO, is crucial in ensuring compliance with local regulations for medical equipment. The increasing worries regarding unknown side effects of AR/VR technologies, particularly in at-risk groups such as children, emphasize the necessity for careful oversight. Nicole Sheynin, a content marketing specialist at AlphaSense, emphasizes the importance of empathy-driven storytelling in navigating these complexities, stating, 'Fueled by empathy-driven storytelling and good coffee, we can better understand the implications of these advancements.'
Additionally, the rise of digital therapeutics and at-home diagnostics during the pandemic illustrates how the market is evolving; the U.S. market for digital therapeutics is projected to grow at a CAGR of 29.8% from 2020 to 2025. The FDA's ongoing initiatives to streamline and modernize the regulatory process, alongside experts like Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and in vitro diagnostics, and Ana Criado, are anticipated to result in quicker review times and more efficient pathways for innovative devices. For manufacturers, staying informed about these trends is critical not only for compliance but also for securing a competitive advantage in a rapidly evolving market.
Conclusion
The evolving landscape of medical device regulation, particularly in relation to De Novo and 510(k) submissions, presents both opportunities and challenges for manufacturers. The 510(k) pathway offers a streamlined route for devices demonstrating substantial equivalence, making it an attractive choice for many. Conversely, the De Novo pathway facilitates the introduction of innovative products that lack a predicate, albeit with a more complex and time-consuming approval process. The insights shared by industry experts underscore the necessity for manufacturers to thoroughly understand these pathways to enhance their market entry strategies and ensure compliance with FDA regulations.
Evaluating the pros and cons of each submission type reveals critical strategic considerations. While the 510(k) process is generally quicker and less burdensome, it may limit innovation for devices without existing predicates. In contrast, the De Novo pathway, while more demanding in terms of documentation and review time, opens doors for novel technologies. As the medical device market continues to evolve, manufacturers must weigh these factors carefully, considering their long-term goals and the competitive landscape.
Looking ahead, upcoming trends such as the integration of real-world evidence and the rise of digital therapeutics are poised to reshape submission protocols and regulatory expectations. Staying abreast of these changes is essential for manufacturers aiming to maintain compliance and secure a competitive edge in a dynamic environment. By adopting a strategic approach and leveraging expert insights, companies can navigate the complexities of medical device regulation effectively, setting the stage for successful product commercialization in a rapidly changing market.




