Overview
The article delineates a five-step process for achieving regulatory compliance in medtech trials in Bolivia. It underscores the critical importance of:
- Comprehending the regulatory framework
- Meticulously preparing required documentation
- Selecting suitable sites
- Effectively recruiting patients
- Ensuring compliance throughout the trial
Each step is bolstered by practical strategies and examples, accentuating the necessity of thorough preparation and ongoing monitoring to adeptly navigate the complexities of local regulations. This structured approach not only facilitates understanding but also empowers stakeholders to address the challenges inherent in clinical research.
Introduction
Navigating the complex landscape of Medtech trials in Bolivia demands a firm grasp of the regulatory framework governing this critical sector. With key bodies like the National Service of Sanitary Control (SENASAG) and the Ministry of Health at the helm, understanding the laws that dictate the registration and commercialization of medical devices is paramount.
As the demand for innovative medical technologies surges, ensuring compliance with both local regulations and international standards becomes increasingly important. This article delves into the essential steps for conducting successful Medtech trials in Bolivia, including:
- Preparing the necessary documentation for regulatory approval
- Implementing effective strategies for site selection
- Patient recruitment
Moreover, it emphasizes the importance of maintaining compliance throughout the trial process, ensuring that all activities align with evolving regulations and ethical standards.
Understand the Regulatory Framework for Medtech Trials in Bolivia
To begin, familiarize yourself with the primary governing organizations overseeing regulatory compliance for medtech trials in Bolivia, such as the National Service of Sanitary Control (SENASAG) and the Ministry of Health. It is essential to review the pertinent laws, including Law No. 1737, which regulates the registration and commercialization of medical devices. A solid grasp of regulatory compliance for medtech trials in Bolivia will empower you to navigate the approval process effectively.
Furthermore, consulting resources like the WHO Global Model Regulatory Framework for Medical Devices can offer valuable insights into international standards that may impact local practices. This foundational understanding is vital for achieving regulatory compliance for medtech trials in Bolivia throughout the testing process.
At bioaccess®, we deliver comprehensive clinical study management services, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Project oversight
Our expertise ensures that your Medtech startup can adeptly navigate the regulatory compliance for medtech trials in Bolivia.

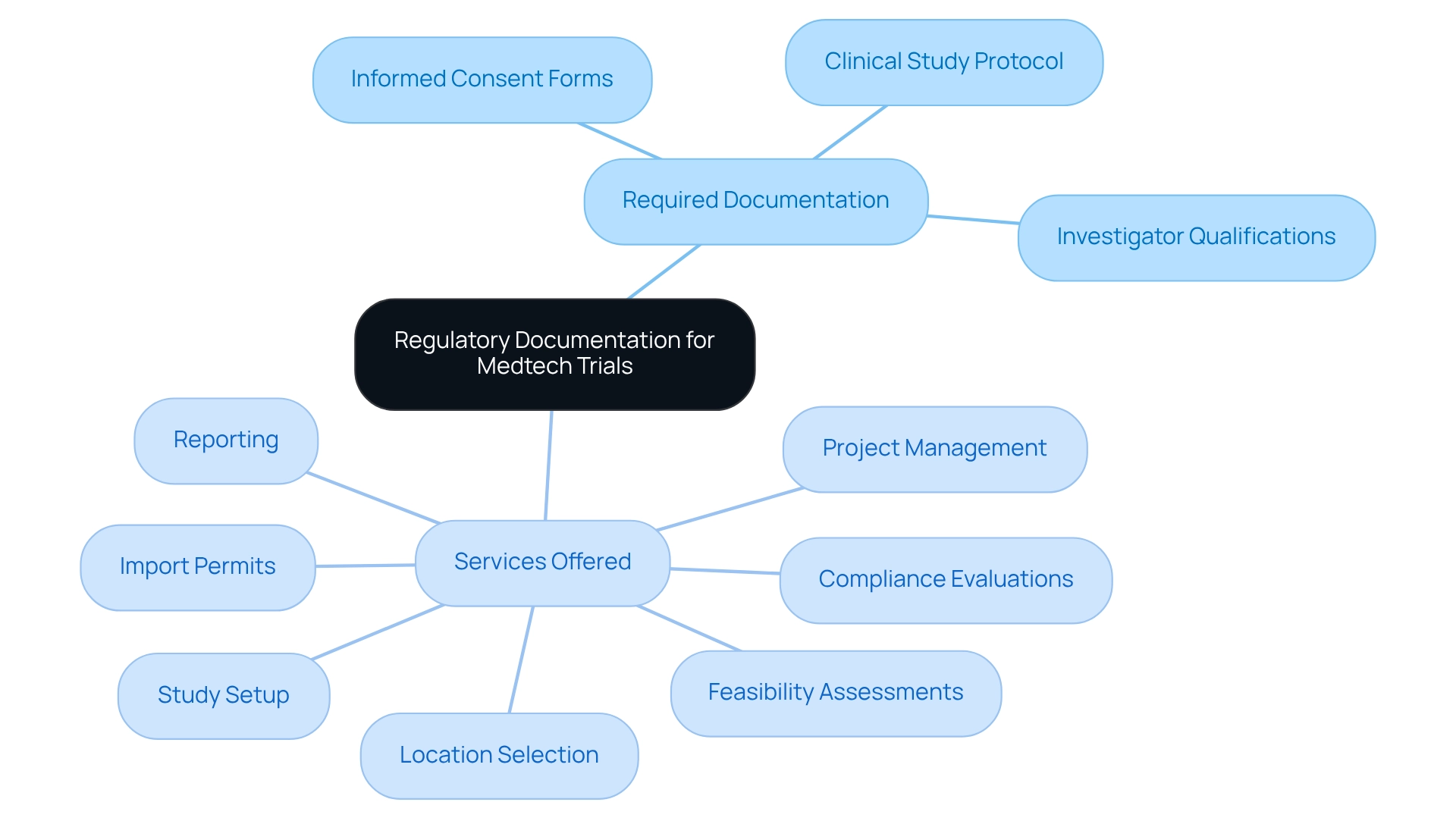
Prepare Required Documentation and Submissions for Regulatory Approval
To achieve regulatory compliance for medtech trials in Bolivia, it is essential to meticulously gather all necessary documentation for successful submissions. This process typically includes:
- A comprehensive clinical study protocol that outlines the study's objectives, methodology, and statistical analysis plan.
- Informed consent forms that comply with ethical standards, ensuring that participants are fully aware of the trial's nature and associated risks.
- Investigator qualifications and location information to effectively showcase the competence of your research team.
It is vital to submit these documents to the appropriate regulatory bodies while ensuring regulatory compliance for medtech trials in Bolivia and adhering to any specific formatting or submission guidelines.
To ensure regulatory compliance for medtech trials in Bolivia, it is necessary to regularly check for updates on submission requirements to avoid delays, as regulations can frequently change.
At bioaccess®, we focus on enabling these processes by providing extensive clinical study management services. Our offerings encompass:
- Feasibility assessments
- Location selection
- Compliance evaluations
- Study setup
- Import permits
- Project management
- Reporting
This ensures a smooth route to market entry in Latin America.
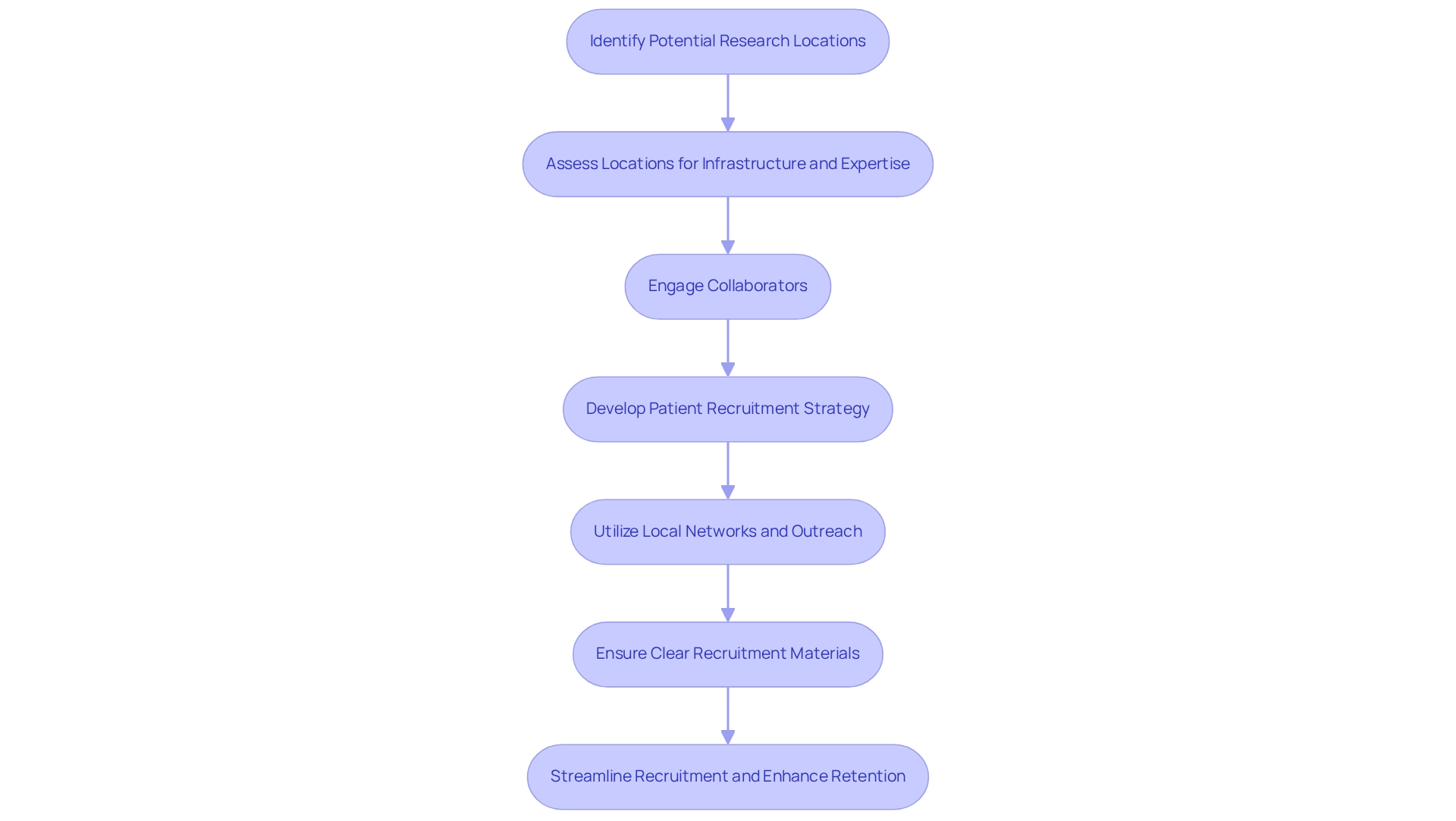
Implement Strategies for Site Selection and Patient Recruitment
To efficiently execute plans for location selection and patient recruitment in Medtech studies, it is essential to recognize potential research locations that possess the required infrastructure and expertise. Collaborations, such as that between bioaccess™ and Caribbean Health Group, significantly enhance the appeal of sites like Barranquilla as leading locations for clinical studies in Latin America.
Assessing locations based on their prior experience with comparable studies, patient demographics, and logistical capabilities is crucial. Once sites are selected, developing a robust patient recruitment strategy becomes imperative. Utilizing local healthcare networks, social media, and community outreach can effectively raise awareness about the study.
Engaging with local stakeholders, including the support of Colombia's Minister of Health, further strengthens recruitment efforts. Additionally, partnering with patient advocacy groups can facilitate reaching potential participants more effectively. It is vital to ensure that recruitment materials are clear and accessible, addressing any concerns prospective participants may have about the study.
This approach not only streamlines recruitment but also enhances retention rates, as evidenced by GlobalCare Clinical Trials' partnership with bioaccess™, which achieved over a 50% reduction in recruitment time and 95% retention rates.
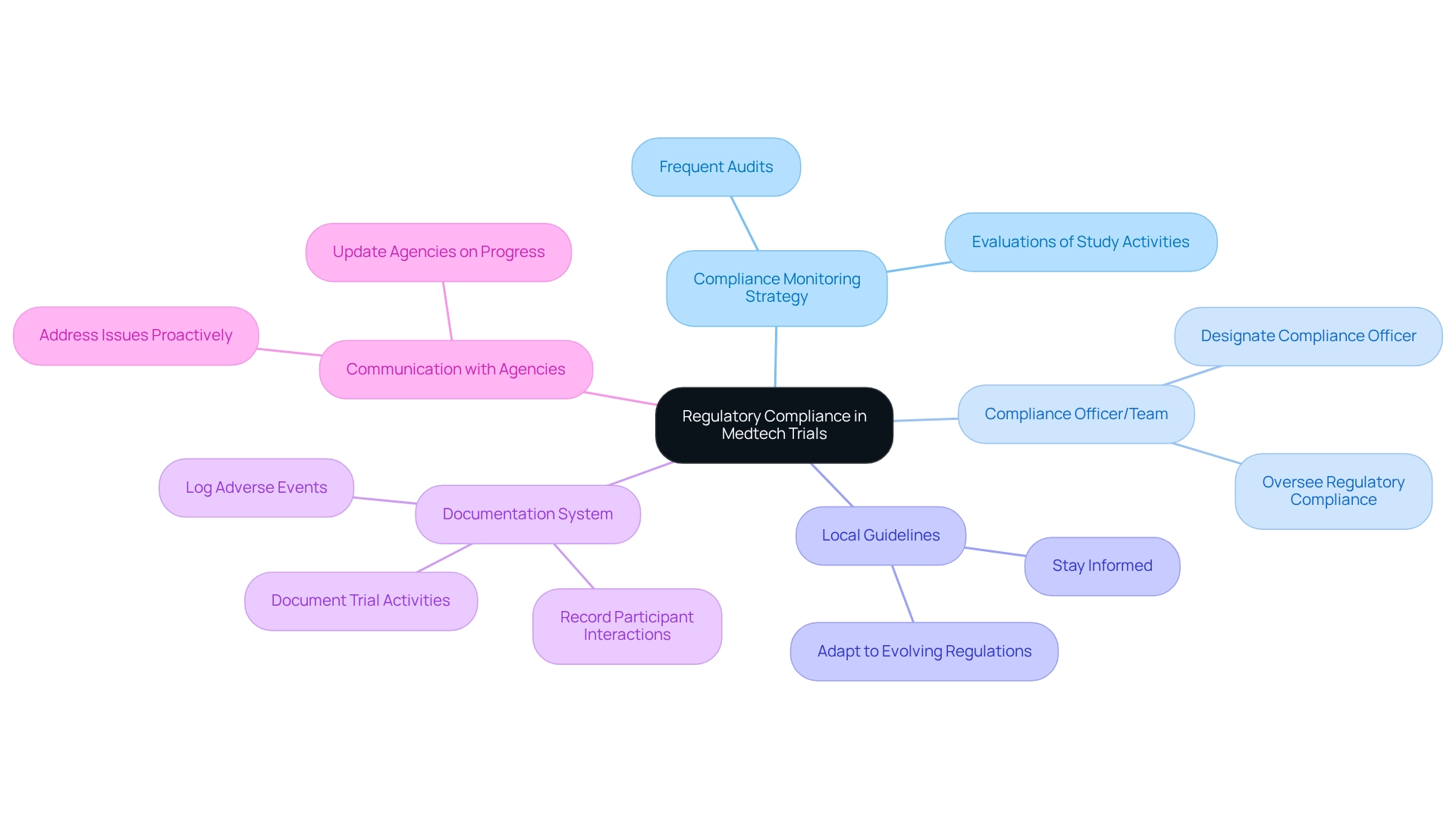
Maintain Compliance and Monitor Regulatory Requirements Throughout the Trial
To ensure regulatory compliance for medtech trials in Bolivia, it is vital to establish a robust compliance monitoring strategy that incorporates frequent audits and evaluations of study activities, ensuring all procedures align with local legal requirements.
- Designate a compliance officer or team tasked with overseeing regulatory compliance for medtech trials in Bolivia, particularly within the dynamic landscape of Latin America, where regulations may frequently evolve.
- Staying informed about local guidelines is essential for regulatory compliance for medtech trials in Bolivia and the successful execution of the experiment.
- Implement a comprehensive system for documenting all trial-related activities, including participant interactions, data collection, and adverse events; this documentation will prove invaluable during audits and inspections.
- Furthermore, maintaining consistent communication with regulatory agencies is crucial. Proactively updating them on progress and addressing any issues fosters a cooperative relationship that can enhance smoother operational processes.
At bioaccess®, we prioritize client trust and information security, ensuring that all data protection measures are implemented to safeguard participant information throughout the clinical research process. Our grievance procedures are designed to transparently address any client concerns while ensuring regulatory compliance for medtech trials in Bolivia, thus reinforcing our commitment to ethical practices in medical device clinical trials.
Conclusion
Successful navigation of Medtech trials in Bolivia is contingent upon a thorough understanding of the regulatory landscape and meticulous execution of essential procedures. Key steps encompass:
- Familiarizing oneself with pivotal regulatory bodies, such as SENASAG and the Ministry of Health.
- Preparing detailed documentation for regulatory submissions.
- Implementing strategic site selection and patient recruitment initiatives.
Each of these elements is critical in ensuring compliance with local regulations and international standards.
Furthermore, vigilant compliance monitoring throughout the trial process is paramount. Establishing a robust compliance framework, which includes:
- Regular audits
- Proactive communication with regulatory bodies
cultivates a culture of accountability and transparency—both vital for the success of clinical trials.
In conclusion, as the demand for innovative medical technologies continues to escalate, so does the necessity of adhering to a structured approach in conducting Medtech trials. By prioritizing regulatory compliance, thorough documentation, and effective recruitment strategies, organizations can strategically position themselves for success within Bolivia's evolving Medtech landscape. Embracing these practices not only enhances the integrity of trials but also propels the advancement of healthcare solutions that can significantly benefit patients and communities alike.
Frequently Asked Questions
What organizations oversee regulatory compliance for medtech trials in Bolivia?
The primary governing organizations are the National Service of Sanitary Control (SENASAG) and the Ministry of Health.
What law regulates the registration and commercialization of medical devices in Bolivia?
Law No. 1737 regulates the registration and commercialization of medical devices in Bolivia.
Why is it important to understand regulatory compliance for medtech trials in Bolivia?
A solid grasp of regulatory compliance empowers individuals to navigate the approval process effectively.
What resources can provide insights into international standards for medtech trials?
The WHO Global Model Regulatory Framework for Medical Devices can offer valuable insights into international standards that may impact local practices.
What services does bioaccess® provide for medtech startups?
Bioaccess® offers comprehensive clinical study management services, including feasibility assessments, site selection, compliance evaluations, study setup, and project oversight.




