Introduction
The journey of medical devices from conception to market is intricately woven with regulatory processes that can significantly impact a manufacturer’s strategy and success. Understanding the distinctions between FDA clearance and approval is essential for navigating these pathways effectively.
- The 510(k) clearance process, designed for devices deemed substantially equivalent to existing products, offers a faster route to market but comes with its own set of challenges, such as documentation and regulatory communication.
- Conversely, the Premarket Approval (PMA) process demands a rigorous evaluation of safety and effectiveness through extensive clinical data, resulting in a more time-consuming and costly pathway.
As the landscape of medical device regulation evolves, manufacturers must grasp these nuances to align their development strategies with regulatory expectations, ensuring timely market access while maintaining compliance.
This article delves into the critical differences between FDA clearance and approval, the steps necessary for achieving either, and the implications of these choices on market access and future trends in medical device regulation.
Understanding FDA Clearance and Approval: Definitions and Implications
The pathways to market for medical products through 510k clearance vs approval are fundamentally different yet crucial for manufacturers to comprehend. FDA Clearance, mainly linked to the 510(k) procedure, allows marketing based on a product's substantial equivalence to an existing, legally marketed item. This pathway prioritizes safety and effectiveness, generally relying on comparative analyses rather than extensive clinical trials.
However, companies frequently encounter challenges such as managing intricate documentation requirements and ensuring timely communication with oversight entities. In contrast, FDA Approval, which is usually linked to the Premarket Approval (PMA) process, mandates a more thorough evaluation of a product's safety and effectiveness through comprehensive clinical data. A recent example is Takeda’s GAMMAGARD LIQUID®, which was approved by the U.S. FDA for adults with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), highlighting the importance of having multiple treatment options for complex diseases.
As Dr. Mamatha Pasnoor, a professor in the Department of Neurology at the University of Kansas Medical Center, states, 'Because CIDP is a progressive and complex disease, multiple treatment options are needed, and clinicians now have an additional therapy that can help adults with CIDP manage their disease.' This distinction between 510k clearance vs approval is essential, as it affects compliance requirements and timelines that manufacturers must navigate, ultimately shaping their market strategies and product development approaches. Recent observations indicate that the average time for FDA 510(k) clearance is significantly shorter than PMA approval, with De Novo wait times rising to 420 days and Panel Track wait times to 289.62 days in 2024.
These increased wait times may pose challenges for companies awaiting official decisions, underscoring the differing challenges faced by companies in bringing their devices to market. Grasping these pathways guarantees that stakeholders can efficiently plan for compliance with regulations and foresee the associated timelines. Moreover, utilizing extensive clinical trial management services, like those provided by bioaccess®, can significantly improve the effectiveness of navigating these compliance procedures.
Bioaccess® specializes in managing a range of studies, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
With their expertise, led by professionals like Katherine Ruiz in Colombia, companies can better align their strategies with regulatory expectations and expedite their journey to market.
Comparing 510(k) Clearance and Premarket Approval (PMA): Key Differences
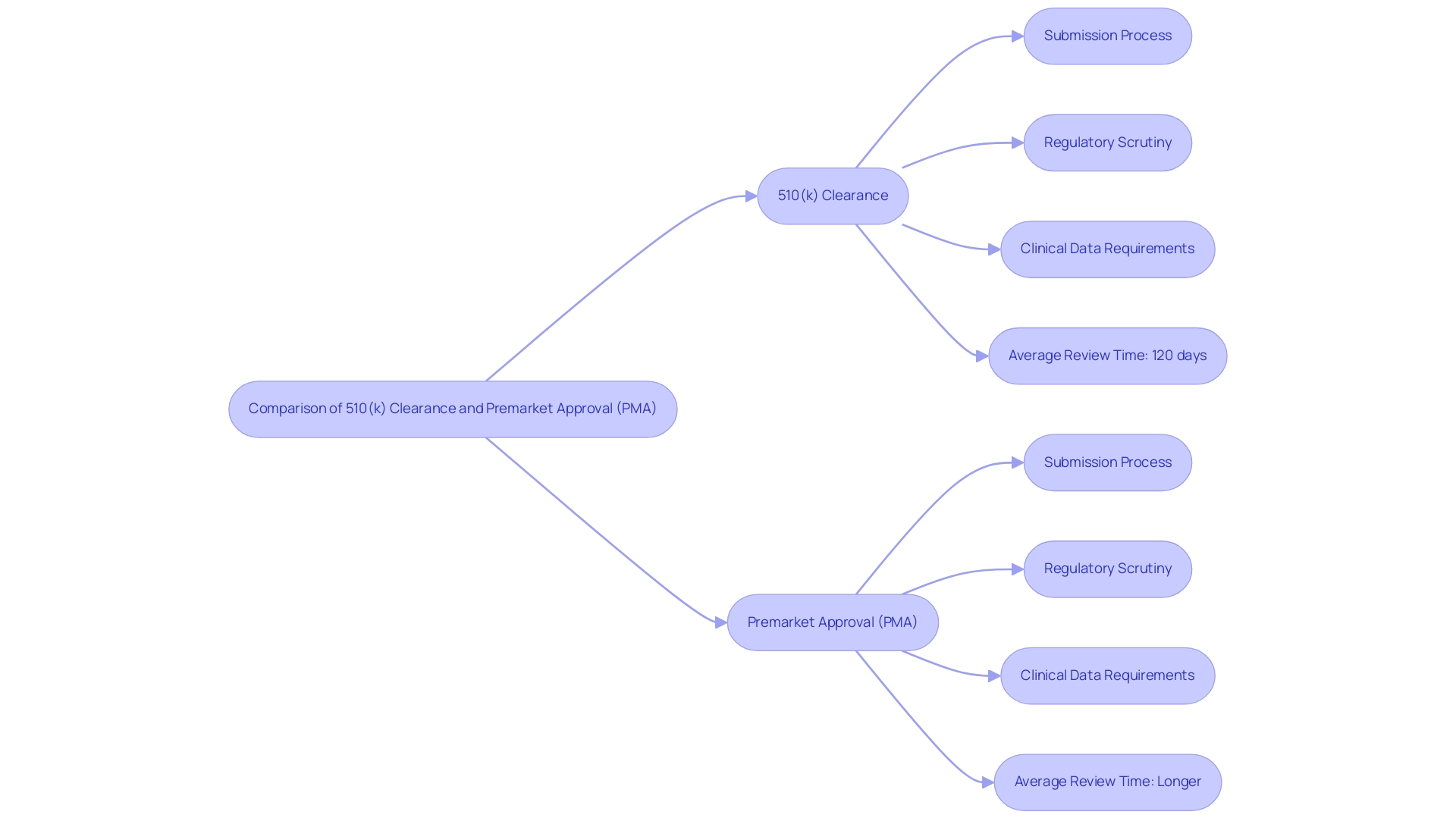
When comparing 510k clearance vs approval, the 510(k) clearance procedure is notably quicker and less resource-intensive than the Premarket Approval (PMA) pathway. Manufacturers must understand the differences between 510k clearance vs approval, as they need to demonstrate that their device is substantially equivalent to an existing device, which generally requires minimal clinical data. This streamlined approach contributes to the high rate of 510k clearance vs approval, with approximately 90% of 510(k) submissions receiving approval from the FDA each year, amidst around 10,000 submissions processed, and an average review time of just 120 days.
In contrast, the PMA process necessitates comprehensive clinical trials to establish both safety and effectiveness, leading to longer timelines and greater costs when considering 510k clearance vs approval. The regulatory scrutiny associated with PMA applications is significantly more rigorous than that of 510k clearance vs approval; these submissions often undergo thorough evaluations, including advisory committee assessments. For instance, a PMA is necessary for high-risk Class III medical devices, which require extensive evidence of safety and effectiveness, including laboratory tests and clinical trials, highlighting the differences between 510k clearance vs approval as discussed in the case study titled 'When to Submit a Premarket Approval (PMA)'.
This rigorous procedure can lead to approval, denial, or requests for additional data, emphasizing the challenges manufacturers face. Recent statistics indicate a slight increase in panel track wait times, rising from 285.80 days in 2023 to 289.62 days in 2024, highlighting the evolving challenges in the approval landscape. However, it is important to note that limitations exist in the study of these processes, including potential inaccuracies in FDA data and the inability to capture all device-related safety concerns.
As companies like bioaccess® demonstrate, understanding and leveraging comprehensive clinical trial management services is essential for navigating these compliance strategies effectively. This encompasses:
- Feasibility studies
- Site selection
- Compliance reviews—which guarantee all study documents adhere to regulatory standards
- Trial setup
- Import permits
- Nationalization of investigational tools
- Project management
- Reporting
As Sebastian Rodriguez-Elizalde, M.D., a Scientific Advisory Board Member, emphasizes, 'Navigating the complexities of medical product approvals requires a thorough understanding of 510k clearance vs approval and PMA processes to ensure compliance and success in the market.
Navigating the Regulatory Landscape: Steps to Achieve FDA Clearance and Approval
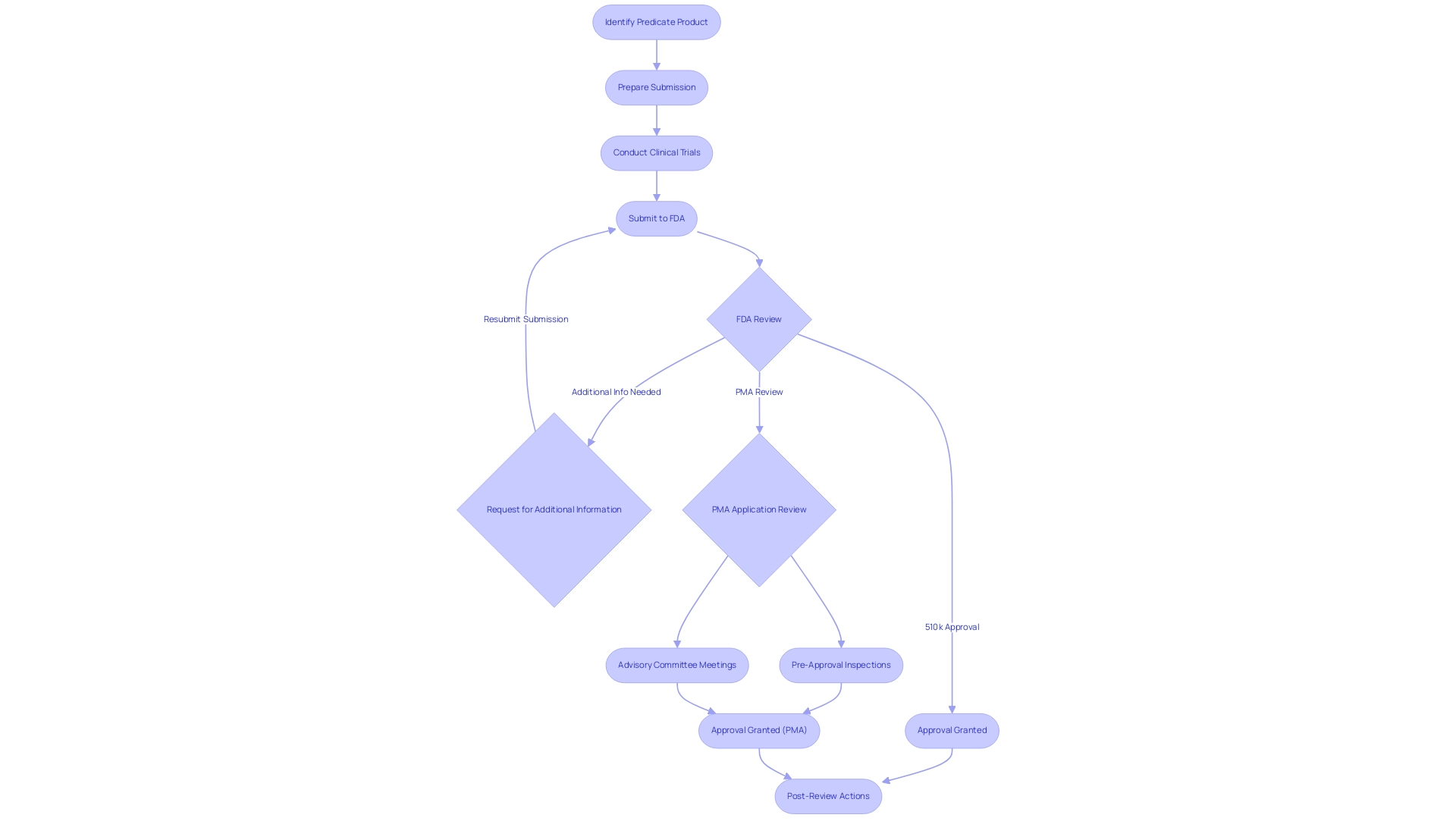
Achieving FDA 510k clearance vs approval necessitates navigating a series of well-defined steps, each tailored to the specific submission pathway. Manufacturers start by identifying a predicate product that serves as a benchmark for comparison in the context of 510k clearance vs approval. This is followed by the preparation of a comprehensive submission that must include detailed descriptions of the apparatus, intended use, and relevant performance data.
In our comprehensive clinical trial management services, we facilitate feasibility studies and site selection to ensure that the right principal investigator (PI) is chosen, and we provide review and feedback on study documents to comply with regulatory requirements. Our expertise encompasses trial setup, start-up, and acquiring necessary approvals from ethics committees and health ministries, including import permits and nationalization of investigational equipment. Once submitted, the FDA conducts a thorough review, which may lead to requests for additional information or clarifications to ensure the safety and efficacy of the product.
In contrast, the procedure of 510k clearance vs approval is more rigorous and multifaceted. Manufacturers are required to conduct clinical trials and compile extensive clinical data that demonstrate the safety and effectiveness of the device. The PMA application undergoes a meticulous review procedure, often involving pre-approval inspections and advisory committee meetings.
As highlighted by Dr. Mat Soukup, Deputy Director of the Division of Biometrics VII,
Rather, it requires careful consideration about the approach to analysis, including topics such as handling of treatment discontinuation, using data from multiple trials, defining summary measures of incidence, and choosing statistical methods to estimate the incidence and corresponding uncertainty.
This underscores the necessity for manufacturers to engage in meticulous planning and strict adherence to FDA guidelines to ensure successful outcomes in their regulatory endeavors. Moreover, when no reference standard is available, it is crucial to report positive percent agreement and negative percent agreement instead of sensitivity and specificity.
Additionally, the FDA suggests reporting results for the intended use population separately from other results to avoid mislabeling specificity. The importance of sensitivity and specificity is further illustrated in the case study titled 'Calculating Sensitivity and Specificity,' which emphasizes their critical role in evaluating diagnostic tests and ensuring accurate estimates. Furthermore, our services encompass comprehensive reporting on study status, inventory, and serious and non-serious adverse events to ensure complete transparency and compliance throughout the clinical trial.
Implications of Choosing 510(k) vs. PMA: Market Access and Legal Considerations
Choosing 510k clearance vs approval can significantly expedite market access, enabling manufacturers to swiftly leverage emerging opportunities. This pathway is particularly appealing due to its relatively streamlined procedure, where the FDA typically communicates its decision within 60 calendar days of receiving a request. A notable example is the INSIGHTEC EXABLATE, which received marketing authorization with submission number P150038 on 07/11/2016, demonstrating successful navigation through the 510(k) pathway.
However, this speed can come with trade-offs, as it may expose manufacturers to legal challenges, particularly when claims of substantial equivalence are contested. In contrast, while the Premarket Approval (PMA) process provides a more comprehensive validation of a product's safety and effectiveness, it subjects submissions to heightened scrutiny and incurs higher costs, especially when considering the 510k clearance vs approval. These factors can significantly impact pricing strategies and the timing of market entry.
Furthermore, the choice between 510k clearance vs approval pathways may influence post-market surveillance obligations and the degree of liability in the event of adverse events. As mentioned by Katrina Rogers, readily available information from the FDA indicates we can anticipate a reasonably high (though not 100%) success rate for our PMA and 510(k) medical submissions. Additionally, as of September 30, 2024, a total of 1,041 Breakthrough Device designations have been granted, indicating a dynamic and evolving landscape in medical device approvals.
Therefore, manufacturers must carefully consider these implications, including the relevant legislation (117 Pub. L. 263), to align their compliance strategy with overarching business objectives, especially as the landscape evolves in 2024. To support these efforts, utilizing comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, project management, and reporting—ensures adherence and effective project management throughout the research and approval stages.
Specialists such as Ana Criado and Katherine Ruiz provide invaluable compliance knowledge that directly improves these services, ensuring a comprehensive understanding of the 510(k) procedure and its requirements.
Future Trends in FDA Pathways: What Lies Ahead for Medical Device Regulation
The terrain of FDA routes for medical instruments is on the verge of notable change, largely shaped by swift technological progress and changing governance philosophies. Recent discussions surrounding regulatory reforms indicate a potential shift toward more efficient processes, particularly for low-risk products, which could favor the debate of 510k clearance vs approval. Furthermore, the emergence of digital health technologies and software as a medical tool (Sand) has prompted the FDA to explore innovative frameworks for evaluation and oversight.
In Colombia, the INVIMA plays a pivotal role as a Level 4 health authority, overseeing medical equipment regulation and ensuring compliance with international standards. A pertinent case study is Artivion's experience with disruptions in its shipping operations due to a ransomware attack, which highlights the critical need for robust cybersecurity measures in the medical device industry. The 2022 Food and Drug Omnibus Reform Act exemplifies this trend, enhancing FDA oversight for remote audits and mandating the inclusion of cybersecurity information in premarket submissions.
Additionally, with experts like Ana Criado, a leader in Regulatory Affairs and CEO of Mahu Pharma, providing insights into clinical trial management, manufacturers can better navigate these complexities. Our service capabilities encompass:
- Feasibility and selection of research locations and principal investigators
- Thorough review and feedback on study documents to guarantee compliance with country requirements
- Comprehensive trial setup and initiation procedures, including ethics committee and health ministry approvals
- Management of import permits and the nationalization of investigational devices
- Ongoing project management and monitoring
Our reporting services cover study status, inventory, and both serious and non-serious adverse events. The FDA has exceeded its pre-submission goal of providing feedback to applicants within 70 calendar days or five days prior to a meeting 75% of the time, demonstrating a commitment to efficiency in its processes. As mentioned by the FDA, 'the RWE/RWD generated through NEST may be utilized not only for post-market surveillance, but it may also be employed to support premarket decision-making and expanded indications for use after 510k clearance vs approval.'
This evolving regulatory landscape necessitates that manufacturers remain vigilant and adaptive, as these trends are likely to influence regulatory requirements and market dynamics in the coming years, demanding agile strategies to ensure compliance and maintain competitiveness.
Conclusion
Navigating the regulatory landscape for medical devices is a complex yet critical endeavor for manufacturers aiming to bring their innovations to market. Understanding the distinctions between FDA clearance and approval is paramount, as these pathways significantly influence timelines, costs, and the overall market strategy.
The 510(k) clearance process, which allows for a quicker route based on substantial equivalence to existing devices, contrasts sharply with the more rigorous Premarket Approval (PMA) process that requires extensive clinical data. This fundamental difference underscores the need for manufacturers to carefully evaluate their options based on their specific device characteristics and market needs.
The implications of choosing between these pathways extend beyond initial market access. Factors such as regulatory scrutiny, potential legal challenges, and post-market obligations can shape a manufacturer’s long-term strategy and risk profile.
As the medical device landscape continues to evolve with advancements in technology and regulatory reforms, manufacturers must remain agile and informed. Leveraging comprehensive clinical trial management services can enhance the efficiency of navigating these regulatory pathways, ensuring compliance and facilitating timely market entry.
As the FDA adapts to emerging trends, including digital health technologies and increased focus on cybersecurity, manufacturers are encouraged to stay abreast of these developments. The future of medical device regulation promises to be dynamic, demanding proactive strategies that align regulatory compliance with business objectives.
By understanding and effectively navigating the complexities of FDA clearance and approval, manufacturers can position themselves for success in an increasingly competitive environment.
Frequently Asked Questions
What is the primary difference between FDA Clearance (510k) and FDA Approval (PMA)?
FDA Clearance, linked to the 510(k) procedure, allows marketing based on a product's substantial equivalence to an existing item, requiring minimal clinical data. In contrast, FDA Approval through the PMA process necessitates comprehensive clinical data to evaluate a product's safety and effectiveness.
How does the timeline for FDA 510(k) clearance compare to PMA approval?
The average time for FDA 510(k) clearance is significantly shorter, with an average review time of about 120 days, while PMA approval can take much longer, with recent panel track wait times reaching approximately 289.62 days.
What challenges do companies face when seeking FDA 510(k) clearance?
Companies often encounter challenges such as managing complex documentation requirements and ensuring timely communication with regulatory entities.
What recent example illustrates the importance of FDA Approval?
Takeda’s GAMMAGARD LIQUID® was approved for adults with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP), highlighting the need for multiple treatment options for complex diseases.
What types of studies does bioaccess® manage to assist companies with compliance?
Bioaccess® manages various studies, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.
Why is it important for manufacturers to understand the distinction between 510k clearance and PMA approval?
Understanding these pathways is crucial as it affects compliance requirements and timelines, ultimately shaping manufacturers' market strategies and product development approaches.
What percentage of 510(k) submissions typically receive approval from the FDA?
Approximately 90% of 510(k) submissions receive approval from the FDA each year, amidst around 10,000 submissions processed.
What additional requirements are associated with the PMA process compared to 510(k) clearance?
The PMA process requires extensive evidence of safety and effectiveness, including laboratory tests and clinical trials, and undergoes more rigorous regulatory scrutiny, including advisory committee assessments.
What is the significance of the increased wait times for PMA approvals noted in recent statistics?
Increased wait times for PMA approvals indicate evolving challenges in the approval landscape, making it essential for companies to plan accordingly for compliance and market entry.
How can comprehensive clinical trial management services benefit companies navigating the FDA approval process?
These services can help streamline compliance strategies, including feasibility studies, site selection, compliance reviews, trial setup, and project management, thereby improving the likelihood of successful market entry.




