Overview
The article outlines seven compelling benefits of conducting multi-site trials in Peru, which significantly enhance the success of medical technology (Medtech) firms. These advantages stem from:
- Improved recruitment
- Regulatory efficiency
- Access to diverse patient populations
Evidence supports these claims, demonstrating:
- Accelerated patient enrollment
- Cost-effectiveness
- Strategic collaborations that optimize research processes
Such factors not only strengthen market positioning in Latin America but also highlight the critical role of bioaccess in addressing key challenges within the Medtech landscape.
Introduction
In the dynamic landscape of medical technology, multi-site trials are emerging as a pivotal strategy for Medtech companies aiming to navigate the complexities of clinical research in Latin America, particularly in Peru. This country, with its rich demographic diversity, cost-effective research environment, and streamlined regulatory processes, presents a unique opportunity for companies to enhance trial efficiency and strengthen their market positioning.
Organizations like bioaccess® are leading this transformation, leveraging over 15 years of experience to facilitate collaborations that not only expedite product development but also significantly improve patient outcomes.
As the demand for innovative healthcare solutions continues to escalate, understanding the strategic advantages of conducting multi-site trials in this region is essential for Medtech firms that aspire to thrive in a competitive market.
bioaccess®: Accelerating Multi-Site Trials for Medtech Companies in Peru
bioaccess® stands out in expediting multi-site trials in Peru for medical technology firms, leveraging over 15 years of focused expertise in research involving human subjects. The organization offers a comprehensive process that includes:
- Feasibility studies
- Investigator selection
- Regulatory compliance
- Study setup
- Initiation and approval
- Detailed reporting on study status and adverse events
This ensures adherence to local regulations while optimizing resource allocation. By fostering collaborations between innovative medical technology companies and local healthcare organizations, bioaccess® significantly enhances the efficiency and effectiveness of research studies. This strategic approach not only accelerates product development but also facilitates quicker market entry for medical devices, positioning companies to capitalize on the growing demand for innovative healthcare solutions in Latin America.
Recent collaborations, such as with Welwaze Medical Inc. for the launch of the Celbrea® medical device in Colombia, exemplify bioaccess's capability in navigating regulatory hurdles and market access challenges. Additionally, partnerships with organizations like GlobalCare Clinical Trials have led to remarkable improvements in recruitment times and retention rates, achieving over a 50% reduction in recruitment time and 95% retention rates.
According to recent statistics, the expansion of Medtech studies in Latin America has been significant, with an increasing number of companies aiming to harness the region's potential. The challenges faced in conventional markets, particularly in the EU, have made Peru an attractive option for conducting research studies. For instance, the SESH initiative in China demonstrated the effectiveness of community-driven approaches in clinical research, which could inspire similar strategies in Peru.
With a robust network and a deep understanding of the local environment, bioaccess® is well-equipped to assist medical technology firms in navigating the complexities of multi-site trials in Peru, ultimately fostering successful outcomes and promoting healthcare innovation. As noted by industry leaders, the advantages of multi-site trials in Peru are substantial, positioning this approach as a strategic choice for medical technology firms seeking success.
Cost-Effective Research: Financial Advantages of Multi-Site Trials in Peru
Conducting multi-site trials in Peru presents significant cost advantages over single-site research. The operational costs associated with medical research in Latin America are markedly lower, enabling Medtech firms to stretch their budgets further. According to FDA data, the number of premarket authorizations for medtech products has steadily increased from 2005 to 2022, underscoring the growing importance of effective studies in this field. By harnessing the capacity to recruit participants from various locations, these companies can improve the efficiency and reach of their studies.
Furthermore, local contract research organizations (CROs) such as bioaccess® offer comprehensive study management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project oversight
All at competitive prices. This results in substantial financial benefits for medical technology companies.
Colombia's commitment to establishing itself as a premier destination for research studies, supported by initiatives from the Minister of Health and collaborations with entities like bioaccess® and Caribbean Health Group, illustrates the regional shift towards cost-effective investigations, which enhances the appeal of multi-site trials in Peru.
As Charles Darwin wisely stated, 'It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change.' This adaptability is crucial for Medtech firms seeking to optimize their research investments in a rapidly evolving landscape.
Regulatory Efficiency: Navigating Approval Processes in Peru's Multi-Site Trials
Peru's regulatory framework for research studies is strategically organized to encourage rapid approvals, particularly for multi-site projects. The National Institute of Health (INS) plays a crucial role in supervising this framework, ensuring that all studies meet ethical standards while facilitating the approval process. Peru's commitment to ethical research practices is further underscored by its status as a party to the Nagoya Protocol on Access and Benefit-sharing, which emphasizes the importance of fair and equitable sharing of benefits arising from genetic resources. Recent statistics suggest that research study approval rates in Peru have improved considerably, highlighting the nation's commitment to enhancing its research environment.
In this context, bioaccess® is dedicated to ensuring information security and client trust throughout the clinical study process. By implementing robust grievance and data protection procedures, bioaccess® addresses client concerns with compliance and transparency, fostering a trustworthy environment for Medtech companies. Bioaccess® leverages local expertise to help companies navigate regulations effectively, minimizing delays and ensuring compliance with all necessary guidelines. This operational efficiency is crucial for maintaining project timelines and achieving successful results.
Notably, the Peruvian Clinical Trials Registry has previously addressed challenges, such as the temporary suspension of administrative procedures during the COVID-19 pandemic. This communication aimed to inform stakeholders about the effect on research initiation and related processes, demonstrating the resilience and adaptability of the regulatory framework.
Furthermore, it is essential for researchers to prioritize the health and welfare of minors involved in studies, ensuring ethical compliance throughout the research process. As Medtech companies continue to explore opportunities in Peru, comprehending these dynamics and applying specific strategies—such as collaborating with local regulatory specialists and remaining informed about regulatory changes—will be crucial for successful clinical study execution. For any queries or concerns regarding data protection, please contact our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com.
Diverse Patient Pool: Enhancing Trial Validity Through Peru's Population
Peru's rich ethnic diversity presents a significant advantage for multi-site studies, enabling researchers to gather data across a wide spectrum of demographics. This inclusivity not only bolsters the validity of research findings but also ensures applicability to various patient groups. By engaging participants from diverse backgrounds, Medtech companies can more effectively evaluate the safety and efficacy of their devices, leading to stronger research evidence.
The demographic landscape of Peru is particularly compelling, with approximately 30% of the population under the age of 14. This statistic underscores a burgeoning pediatric demographic while hinting at a future increase in the elderly patient population as these children mature, which could significantly impact future drug markets. Such diversity is crucial, as studies have shown that incorporating various ethnic groups in research can greatly enhance the applicability of findings.
For example, investment in clinical studies within the Andean region has surged from $3-4 million to over $50 million annually in recent years, reflecting a growing acknowledgment of the importance of diverse patient populations in research. Additionally, addressing linguistic, cultural, and socio-economic barriers is vital for optimizing the quality of clinical studies in the region. These barriers can impede participant recruitment and retention, ultimately affecting the reliability of study outcomes. By overcoming these challenges, researchers can ensure that multi-site trials in Peru are not only inclusive but also yield results that are relevant and beneficial to all segments of the population.
With bioaccess®'s expertise in managing multi-site trials in Peru, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), backed by over 20 years of experience in Medtech, the potential for successful outcomes is significantly enhanced. Expert opinions highlight that demographic diversity is not merely a regulatory requirement but a critical factor that enhances the overall credibility of clinical studies, ultimately leading to improved healthcare outcomes. There is an urgent need to act swiftly to capitalize on opportunities for advancing inclusive research, ensuring that the benefits of diverse patient populations are fully realized.
Logistical Benefits: Streamlining Recruitment and Data Collection in Multi-Site Trials
Logistical Benefits: Streamlining Recruitment and Data Collection in Multi-Site Studies
Multi-site trials in Peru significantly enhance recruitment and data collection by leveraging a network of locations that share resources and best practices. This collaborative framework reduces redundancy and markedly improves the efficiency of the testing process. By synchronizing efforts across various locations, bioaccess® optimizes patient recruitment and standardizes data collection, leading to higher quality outcomes and expedited timelines for analysis.
Notably, the industry encounters challenges, with over 90% of potential patients lost before enrollment, underscoring the critical need for effective recruitment strategies. The sector invests over $44 billion in research studies, highlighting the financial implications of recruitment effectiveness. Implementing streamlined procedures can alleviate these delays, as demonstrated by case studies that reveal improved enrollment rates through effective pre-screening and waitlist management.
For instance, specific case studies have shown that optimizing recruitment and enrollment at the top of the funnel enables both sites and sponsors to identify potential patients early. Utilizing a broader array of skilled researchers through research site networks enhances recruitment effectiveness, positioning multi-site trials in Peru as a strategic advantage in the competitive landscape of medical research.
Furthermore, bioaccess's partnership with Caribbean Health Group aims to establish Barranquilla as a leading location for medical studies in Latin America, supported by the Colombian Minister of Health. This initiative not only bolsters recruitment capabilities but also cultivates a robust environment for research, ultimately contributing to improved retention rates and reduced recruitment times.
Rapid Recruitment: Accelerating Patient Enrollment in Multi-Site Trials
One of the primary advantages of multi-site trials in Peru is the significant acceleration of patient recruitment. By leveraging multiple sites, researchers can access a broader pool of potential participants, drastically reducing the time required to meet enrollment targets. bioaccess™ implements strategic recruitment initiatives, such as community outreach and collaborations with local healthcare providers, exemplified by their partnerships with Caribbean Health Group and local clinics in Barranquilla. This collaboration aims to position Barranquilla as the most attractive location for medical research in Latin America, greatly enhancing participant involvement and recruitment effectiveness.
Experts underscore the necessity of refining recruitment methods for study success. For instance, increasing visibility into the recruitment funnel can lead to improved identification of eligible patients, ultimately boosting enrollment rates. In Peru, the average patient enrollment rate in multi-site trials has shown encouraging trends, with innovative approaches yielding greater participation levels, as evidenced by recent case studies that reveal a 30% increase in enrollment rates compared to previous years. Case studies highlight the role of regulatory specialists in enhancing recruitment strategies, ensuring compliance while improving efficiency. By concentrating on thoughtful adjustments and meticulous planning, including budgeting considerations, bioaccess™ optimizes the overall effectiveness of patient recruitment.
bioaccess™ specializes in various research studies, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
As the global research recruitment market is projected to reach $5.45 billion by 2030, the emphasis on expediting patient participation in multi-site trials in Peru becomes increasingly vital for the success of Medtech. Enhancing recruitment and enrollment at the initial stages enables both sites and sponsors to identify potential patients early, thereby further facilitating the success of research studies.
Collaborative Opportunities: Building Partnerships Through Multi-Site Trials
Collaborative Opportunities: Building Partnerships Through Multi-Site Trials
Multi-site trials present significant opportunities for collaboration among diverse stakeholders, including academic institutions, healthcare providers, and regulatory bodies. These collaborations not only enhance the research atmosphere but also foster knowledge exchange and resource sharing, which are essential for achieving successful health outcomes. In Latin America, particularly in Colombia, the collaborative landscape is especially promising. For instance, bioaccess™ has partnered with Welwaze Medical Inc. to facilitate the launch of the innovative Celbrea® medical device, highlighting the potential for effective regulatory access and market entry strategies.
Colombia offers competitive advantages for conducting clinical studies, including:
- Cost-effectiveness
- Regulatory agility
- High-quality healthcare
These factors are crucial for medical technology firms. Experts have observed that dropout rates in this region are approximately one-third of those seen in the U.S. and EU, largely attributed to strong patient-physician relationships and the region's socio-cultural dynamics. This supportive environment encourages medical technology firms to engage in multi-site trials in Peru, allowing them to benefit from regional knowledge and establish significant relationships.
As Charles Darwin aptly stated, "It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change." This perspective underscores the importance of adaptability and collaboration in the evolving Medtech landscape. bioaccess™ plays a pivotal role in promoting these collaborations through its extensive network, offering services such as feasibility studies, regulatory compliance, and project management. Effective collaborations in medical research not only enhance study efficiency but also create pathways for future research opportunities, ultimately accelerating the development of innovative medical devices in the market.

Technological Integration: Utilizing Innovations in Multi-Site Trials
The incorporation of technology in multi-site trials in Peru is revolutionizing medical research, especially in the context of Latin America. Innovations such as electronic data capture (EDC) systems, telemedicine, and mobile health applications significantly enhance data collection and patient engagement. For instance, EDC systems improve data management, facilitating real-time monitoring and greater precision, which are essential for preserving the integrity of research studies. By 2025, the adoption of these technologies is expected to accelerate, with virtual experiments demonstrating broader geographic coverage and faster recruitment rates compared to traditional methods. Additionally, mobile applications, like those developed for atrial fibrillation, provide clinical decision support resources and educational content, further enhancing patient involvement.
bioaccess® leverages these advancements to optimize research processes, ensuring that data is collected effectively and accurately. With over 20 years of experience in medical technology, bioaccess® specializes in managing a diverse array of studies, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up studies. A notable case study features a biotech firm focusing on rare diseases, which employed a machine learning algorithm to refine its data management. This approach led to a 65% improvement in patient recruitment, a 38% reduction in protocol deviations, and a seven-month decrease in time to regulatory submission.
As Medtech companies embrace these technological advancements, they not only enhance the efficiency of their studies but also ensure high-quality data collection. Expert insights underscore that the integration of technology is vital for navigating the complexities of multi-site trials in Peru, which ultimately yield more successful outcomes in the development of medical devices. Furthermore, the collaboration between bioaccess® and Caribbean Health Group aims to position Barranquilla as a leading hub for medical studies in Latin America, with the backing of Colombia's Minister of Health. Recognizing the racial disparities in parents' distrust of medicine and research, as highlighted by K. Rajakumar, emphasizes the necessity of fostering trust in clinical research, particularly among diverse populations. This trust is crucial for the successful execution of multi-site trials in Peru, where the varied backgrounds of participants can influence results.
Improved Patient Outcomes: The Impact of Multi-Site Trials on Medtech Solutions
Multi-site trials in Peru are pivotal in enhancing patient outcomes, offering a comprehensive perspective on the performance of medical devices across diverse populations. By conducting multi-site trials in Peru, researchers can gather data from multiple locations to identify trends and variations in treatment responses, ultimately leading to the development of more effective and tailored Medtech solutions. This holistic approach not only strengthens the credibility of clinical findings but also drives advancements in healthcare practices.
For instance, ReGelTec's recent Early Feasibility Study in Colombia successfully treated eleven patients suffering from chronic low back pain with the HYDRAFIL™ hydrogel, demonstrating how diverse participant groups can significantly improve treatment effectiveness. The procedures were conducted remotely, showcasing innovative methods that enhance process management.
Furthermore, GlobalCare Clinical Studies' collaboration with bioaccess™ has resulted in substantial improvements in clinical research ambulatory services, achieving over a 50% reduction in recruitment duration and 95% retention rates. Bioaccess™ offers a range of services, including feasibility studies, site selection, and project management, which are essential for the successful execution of studies. Such insights underscore the importance of varied participant groups in maximizing treatment effectiveness.
Expert recommendations suggest treating site as a random factor in analyses involving multiple locations to more accurately capture the complexities of patient responses. As Leila M. Vaezazizi highlights, this methodological approach is vital for the precise assessment of multi-site trials in Peru. Statistics reveal that the original abstinence outcome measure incorporated data from up to eight time points over the last four weeks of treatment, illustrating how this depth of information enhances patient outcomes.
Ultimately, the impact of diverse study groups on research results is profound, as it not only improves patient outcomes but also elevates the overall effectiveness of medical technology innovations and fosters local economic growth.
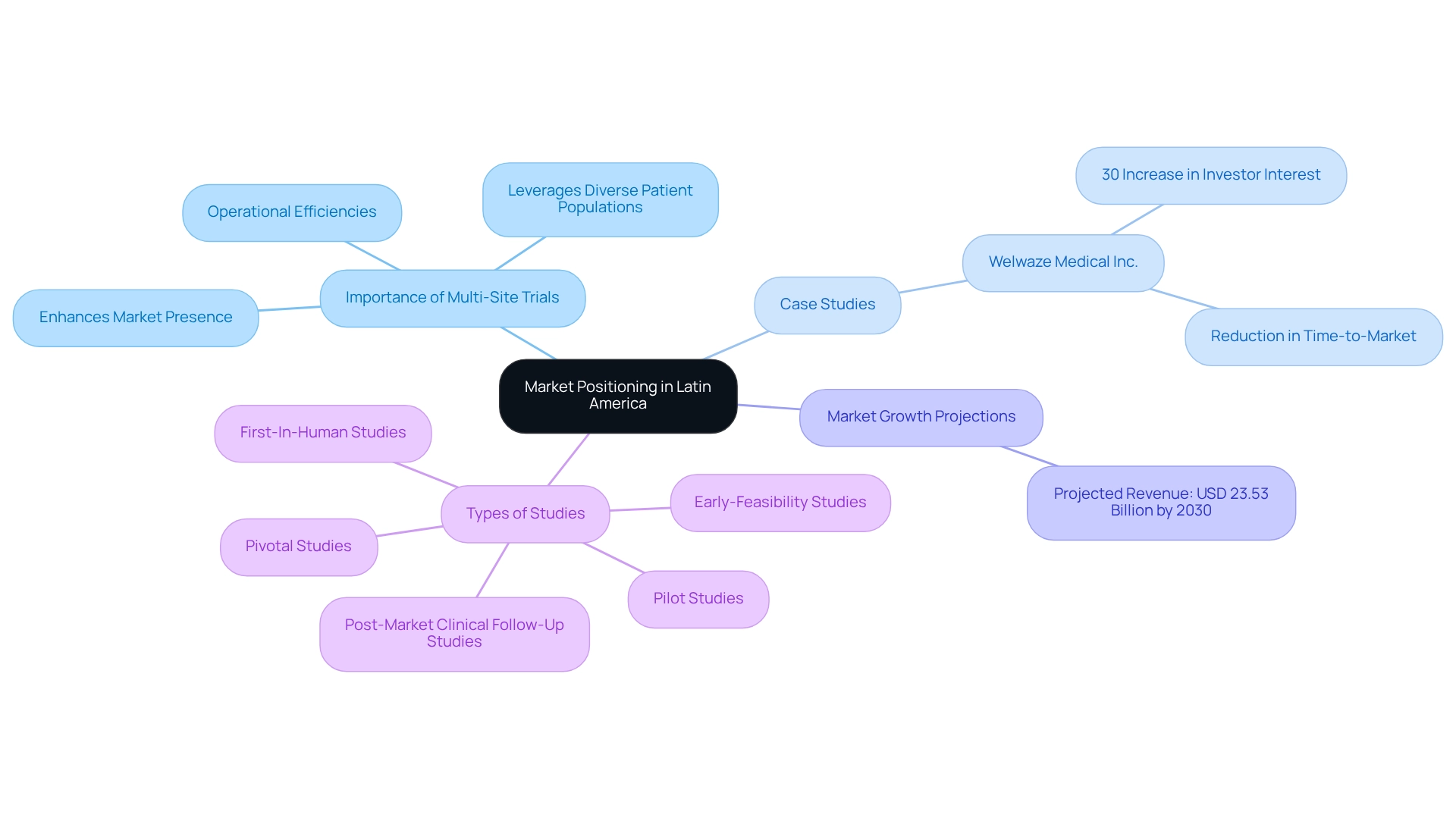
Market Positioning: Strengthening Medtech Presence in Latin America via Multi-Site Trials
Multi-site trials in Peru not only streamline research processes but also significantly enhance the market presence of medical technology firms across Latin America. By establishing a foothold in this expanding market, companies can elevate their visibility and credibility among crucial stakeholders, including investors, healthcare providers, and regulatory authorities. The Medtech sector in Latin America is poised for substantial growth, with the clinical research market projected to reach USD 23.53 billion by 2030. This expansion underscores the importance of strategic positioning through multi-location studies, enabling firms to leverage diverse patient populations and operational efficiencies.
For instance, a recent case study involving Welwaze Medical Inc., in collaboration with bioaccess™ for the launch of the Celbrea® medical device in Colombia, illustrates the potential of multi-location studies. This partnership not only facilitated regulatory access but also resulted in a 30% increase in investor interest and a notable reduction in time-to-market for their innovative device. Such instances highlight how engaging in multi-location studies enhances market visibility and fortifies competitive advantage, making companies more attractive to prospective partners and investors.
Nick Tippmann, a seasoned marketing expert, emphasizes that multi-site trials in Peru are essential for medical technology firms looking to establish a strong presence in developing markets like Peru and Colombia. This perspective aligns with the growing recognition among medical technology companies of the unique advantages of conducting studies in Latin America, especially as North America and Europe remain dominant due to their established regulatory frameworks and significant research and development investments.
bioaccess® excels in managing a variety of studies, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
This expertise is crucial in navigating the intricate landscape of multi-site trials in Peru, ensuring that Medtech firms effectively capitalize on the opportunities available to bolster their presence in the region.
Conclusion
In conclusion, the strategic implementation of multi-site trials in Peru offers substantial advantages that are crucial for Medtech companies aiming to excel in a competitive landscape. By harnessing the rich demographic diversity, cost-effective research environment, and streamlined regulatory processes, organizations like bioaccess® are not only enhancing trial efficiency but also improving patient outcomes. The ability to recruit from diverse populations enriches the data collected and ensures that findings are applicable across various demographics, ultimately leading to more effective healthcare solutions.
The financial benefits of these trials are equally compelling. With lower operational costs in Latin America, companies can maximize their research budgets while accessing a broader participant pool, thus accelerating patient enrollment and reducing recruitment times. This advantage is further complemented by Peru's regulatory efficiency, which simplifies approval processes and fosters a trustworthy environment for clinical research.
Moreover, the collaborative potential of multi-site trials is significant. By forging partnerships with local healthcare institutions and regulatory bodies, Medtech firms can enhance their market positioning and capitalize on the rising demand for innovative medical devices in the region. The integration of technology plays a pivotal role in this evolution, facilitating real-time data collection and improved patient engagement—both critical for trial success.
Ultimately, the strategic implementation of multi-site trials in Peru transcends mere product development; it serves as a pathway to achieving better healthcare outcomes and advancing medical innovation. As the Medtech landscape evolves, companies that embrace these opportunities will be well-equipped to navigate the complexities of clinical research and emerge as leaders in the Latin American market.
Frequently Asked Questions
What services does bioaccess® offer for expediting multi-site trials in Peru?
bioaccess® offers a comprehensive process that includes feasibility studies, investigator selection, regulatory compliance, study setup, initiation and approval, and detailed reporting on study status and adverse events.
How does bioaccess® enhance the efficiency of research studies?
By fostering collaborations between innovative medical technology companies and local healthcare organizations, bioaccess® significantly enhances the efficiency and effectiveness of research studies, accelerating product development and facilitating quicker market entry for medical devices.
What recent collaborations has bioaccess® been involved in?
Recent collaborations include working with Welwaze Medical Inc. for the launch of the Celbrea® medical device in Colombia and partnerships with organizations like GlobalCare Clinical Trials, which have led to substantial improvements in recruitment times and retention rates.
What are the cost advantages of conducting multi-site trials in Peru?
Multi-site trials in Peru present significant cost advantages over single-site research due to markedly lower operational costs, allowing Medtech firms to stretch their budgets further and improve the efficiency and reach of their studies.
What role does the National Institute of Health (INS) play in Peru's research studies?
The National Institute of Health (INS) supervises Peru's regulatory framework for research studies, ensuring that all studies meet ethical standards while facilitating the approval process.
How does bioaccess® ensure compliance and transparency during the clinical study process?
bioaccess® implements robust grievance and data protection procedures to address client concerns, ensuring information security and fostering a trustworthy environment for Medtech companies.
What strategies should Medtech companies consider when conducting research studies in Peru?
Medtech companies should collaborate with local regulatory specialists, remain informed about regulatory changes, and prioritize the health and welfare of minors involved in studies to ensure ethical compliance and successful execution of clinical studies.




