Overview
The article centers on the essential components of post-market device trial design, underscoring the necessity of rigorous evaluations to guarantee ongoing patient safety and device effectiveness. It asserts that comprehending regulatory requirements, utilizing diverse research designs, and prioritizing ethical standards are vital for manufacturers. These elements are crucial for navigating the complexities of post-market assessments and enhancing medical technology outcomes. In the ever-evolving Medtech landscape, understanding these challenges is paramount for driving innovation and ensuring the safety of patients. The collaboration among stakeholders is essential to address these challenges effectively and to improve the overall landscape of medical technology.
Introduction
In the ever-evolving landscape of medical technology, the significance of post-market trials is paramount. These studies are essential for monitoring the safety and effectiveness of medical devices once they have entered the market. By collecting real-world data, post-market trials identify unforeseen issues, ensure patient safety, and provide insights that inform future regulatory decisions.
As the industry faces increasing regulatory scrutiny, particularly with the implementation of the Medical Device Regulation (MDR) in 2025, understanding the objectives, types, and design considerations of these trials becomes crucial for manufacturers seeking to maintain compliance and foster innovation.
This article explores the fundamental aspects of post-market trials, highlighting their importance, regulatory requirements, and the pivotal role they play in safeguarding both patients and the integrity of medical devices.
Understanding Post-Market Trials: Importance and Objectives
The continuous evaluation of the safety and effectiveness of medical devices post-market hinges on well-designed trial structures. These studies are pivotal in gathering real-world data, essential for uncovering unforeseen issues that may surface during routine use. The primary objectives of after-market studies are to ensure ongoing patient safety, evaluate long-term performance, and generate insights that can guide future regulatory decisions.
In 2025, the importance of these trials is underscored by the stringent requirements of the Medical Device Regulation (MDR), which necessitates rigorous monitoring after market release to uphold CE marking for devices. This compliance not only protects patient safety but also fosters business growth and innovation, enabling manufacturers to enhance their products based on real-world insights. Bioaccess® excels in managing post-market clinical follow-up research (PMCF), alongside Early-Feasibility Research (EFS), First-In-Human Research (FIH), Pilot Research, and Pivotal Research, ensuring that manufacturers remain compliant with these vital regulations.
A case analysis titled "Consequences of Non-Compliance with PMS Regulations" revealed that manufacturers neglecting PMS regulations faced severe repercussions, including regulatory non-compliance, fines, and product recalls. This situation underscores the critical necessity for adherence to these regulations, safeguarding both patients and manufacturers alike.
Statistics indicate that post-marketing research is crucial for evaluating the relevance of premarketing data in real-world settings. For example, the percentage of patients using warfarin in the target population was a mere 1.0%, compared to 3.8% in the broader population. This disparity emphasizes the need for ongoing assessments of device performance across varied patient demographics, reinforcing the significance of post-marketing research.
Expert insights, such as those from Professor Yang Wang, highlight the importance of statistical considerations in follow-up evaluations. He notes that the emphasis on sample size, research design, and statistical methodologies varies to address the unique objectives and challenges of each phase of clinical evaluations. This complexity illustrates the intricate nature of post-market device trial design, aimed at producing effective studies after market introduction.
With over 20 years of experience in Medtech, bioaccess® is exceptionally equipped to navigate the complexities of after-launch studies in Latin America. Real-world examples demonstrate how follow-up studies can significantly improve patient outcomes. By collecting and analyzing real-world data, stakeholders can gain a deeper understanding of the long-term effects of medical devices, ultimately fostering greater public confidence in medical technologies.
Understanding these objectives allows stakeholders to appreciate the crucial role that follow-up evaluations play in the lifecycle of medical products, ensuring that advancements consistently meet the needs of both patients and healthcare providers.
Navigating Regulatory Requirements for Post-Market Trials
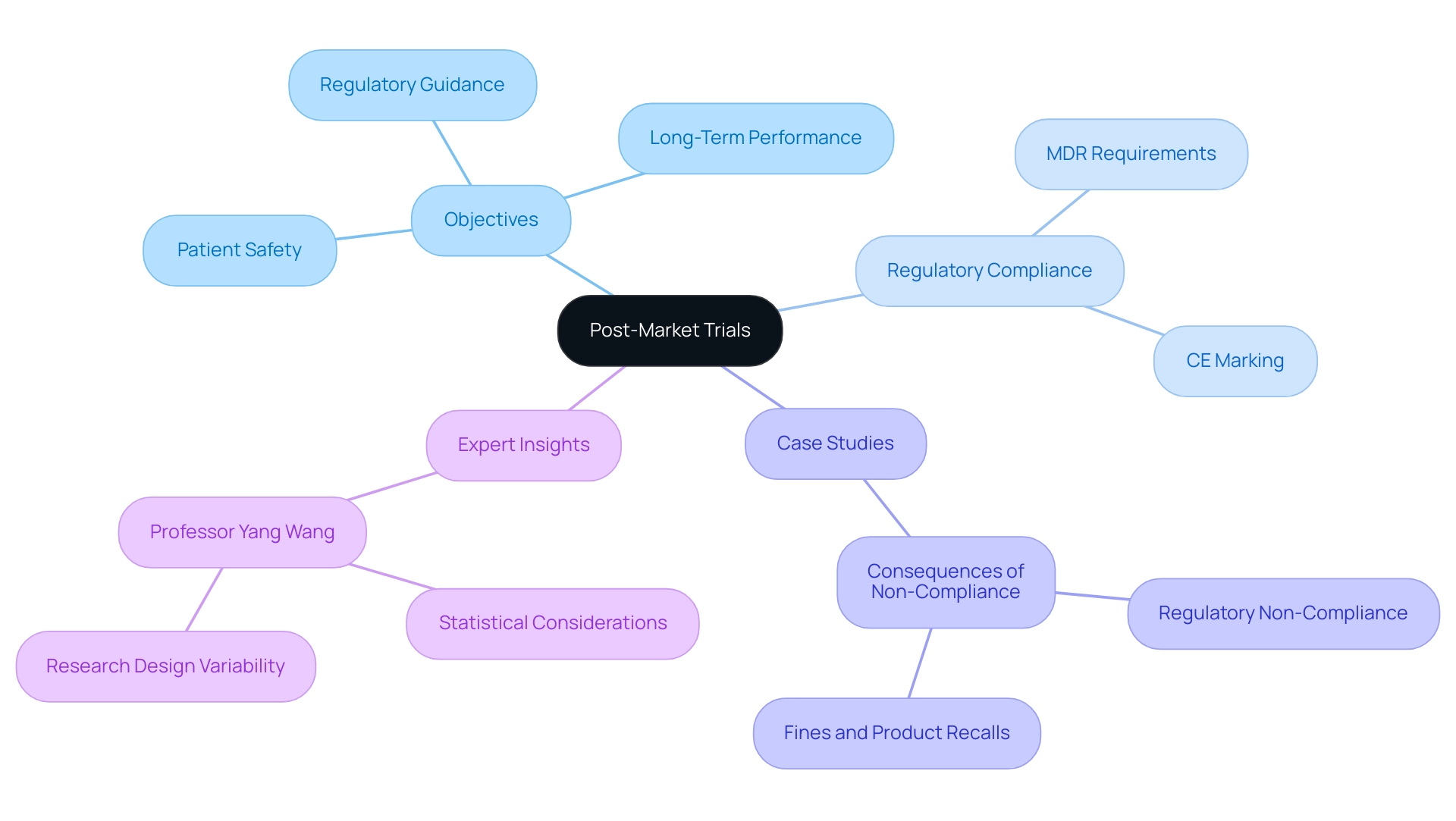
Post-market trials are governed by a complex framework of regulatory requirements that vary across regions, with the United States being a key market. The FDA mandates that producers conduct evaluations after market release to consistently assess the effectiveness and safety of medical equipment. Central to these regulations are the Medical Device Reporting (MDR) requirements, which obligate manufacturers to promptly report any adverse events and device malfunctions. This ensures that potential risks are swiftly identified and addressed.
Moreover, manufacturers are required to develop a comprehensive Post-Market Surveillance (PMS) plan. This plan must detail the methodologies for data collection and analysis, ensuring that the information gathered is both systematic and actionable. Compliance with these regulatory requirements is not merely a legal obligation; it is crucial for maintaining product integrity and safeguarding patient safety.
As of 2025, the FDA has established an automated tracking system to enhance the oversight of ongoing surveillance efforts, ensuring that producers meet their monitoring obligations promptly. This initiative reflects the FDA's commitment to regulatory oversight and underscores the significance of studies conducted after market release in the Medtech sector. A notice of availability for the draft guidance on postmarket surveillance appeared in the Federal Register on May 27, 2021, providing historical context for the current regulations.
Lauren K. Roth, Associate Commissioner for Policy, stated, "The Food and Drug Administration (FDA, Agency, or we) is announcing the availability of the final guidance documents entitled 'Postmarket Surveillance Under Section 522 of the Federal Food, Drug, and Cosmetic Act' and 'Procedures for Handling Post-Approval Studies Imposed by PMA [Premarket Approval Application] Order." Understanding these evolving requirements is essential for manufacturers aiming to navigate the regulatory landscape effectively and leverage the full potential of their medical devices in the market.
bioaccess® plays a pivotal role in this landscape by supporting Medtech startups in navigating the complexities of post-market device trial design. Our comprehensive services encompass:
- Feasibility and selection of research locations
- Investigator selection
- Review and feedback on research documents
- Regulatory compliance
- Project management
- Reporting on research status and adverse events
We ensure that our clients meet these regulatory requirements while advancing their innovative medical products.
Additionally, bioaccess® is committed to ensuring information security and client trust, addressing any concerns through our dedicated Grievance and Data Protection Officer, thereby fostering transparency and compliance in all our processes.
Types of Studies in Post-Market Device Trials
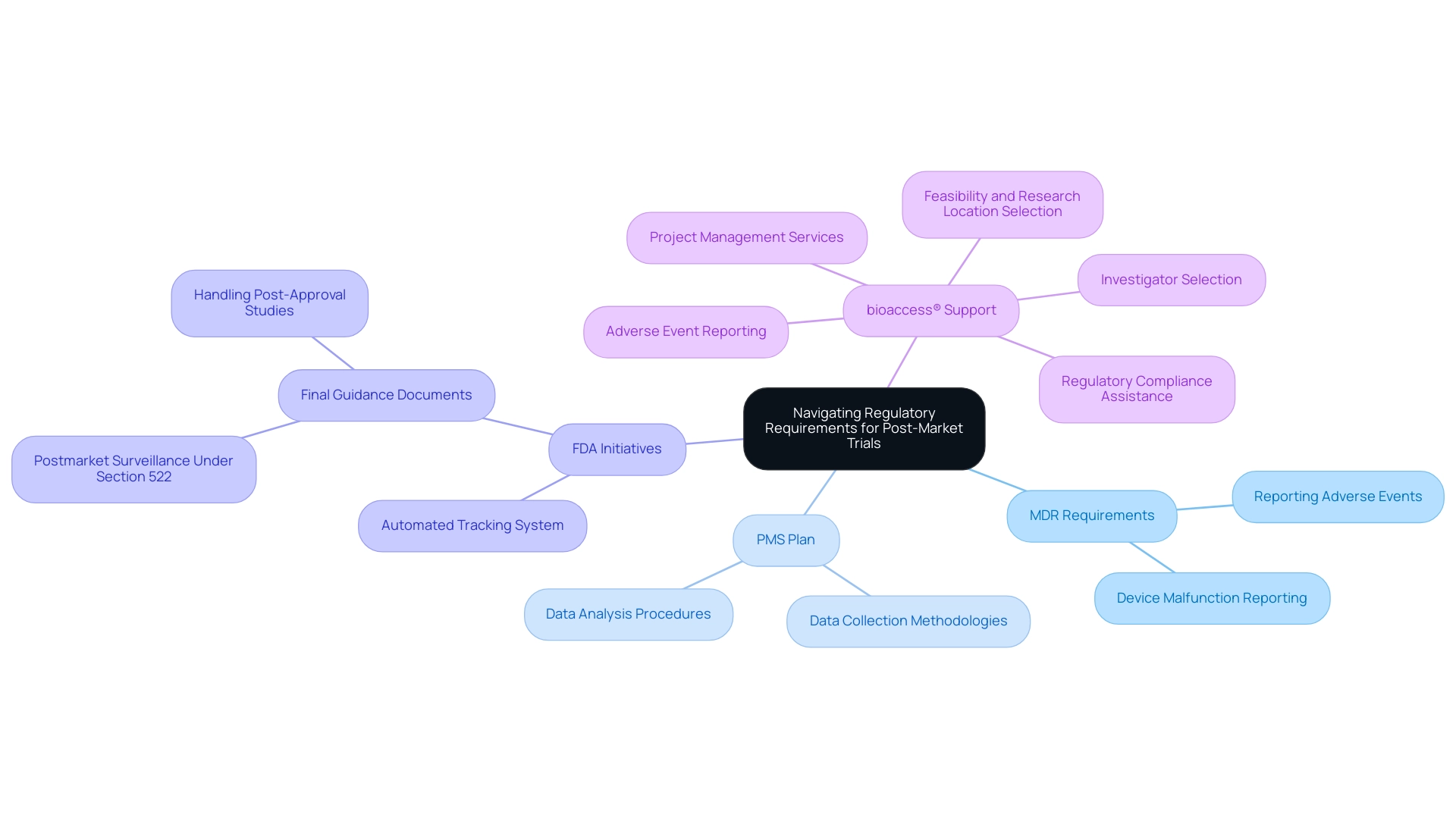
Post-market device trial design represents a critical component of post-market assessments, fulfilling distinct roles in the evaluation of medical products once they have entered the market. Key research designs, including observational research, randomized controlled trials (RCTs), and registry research, can be effectively managed by bioaccess®'s comprehensive clinical trial management services, backed by over 20 years of experience in Medtech.
- Observational Studies: These studies collect information on performance in real-world environments without researcher intervention. They are vital for understanding how instruments function across diverse patient groups and can reveal long-term outcomes that may not be evident in regulated settings.
- Randomized Controlled Trials (RCTs): Often regarded as the gold standard in clinical research, RCTs compare the device against a control group, which may receive a placebo or an existing standard-of-care treatment. This design is instrumental in establishing the efficacy and safety of the device under controlled conditions, providing robust evidence for regulatory submissions and market acceptance.
- Registry Research: These investigations involve the systematic collection of information from a defined population over time, focusing on long-term outcomes and device performance. Registries can yield insights into the effectiveness of devices in routine clinical practice and help identify rare adverse events that may not be captured in smaller studies.
Each research type possesses its own strengths and weaknesses. For example, while observational studies can provide insights into real-world effectiveness, they may lack the rigor of RCTs, which are designed to minimize bias. Conversely, RCTs can be resource-intensive and may not always reflect real-world usage.
As we look toward 2025, the landscape of post-market device trial design continues to evolve, emphasizing proactive risk mitigation strategies. Such methods are essential for ensuring that medical products remain on the market longer by addressing potential safety concerns early on. Expert opinions suggest that a balanced approach, utilizing both observational studies and RCTs within post-market device trial design, can yield comprehensive insights into performance, ultimately benefiting both manufacturers and patients.
As noted by Sumatha Kondabolu, a QA/RA expert with 19 years of experience, "Comprehending the subtleties of evaluations after market release is essential for guaranteeing product safety and effectiveness."
Statistics underscore the importance of post-market surveillance (PMS), particularly for devices intended to support or sustain life outside of user facilities, highlighting the necessity of rigorous evaluation in these contexts. For instance, Phase III studies validate a tool's safety, effectiveness, and benefits in a larger patient cohort, comparing it to current therapies or standard-of-care devices. Expert management of multi-center studies by bioaccess® ensures efficient enrollment and high-quality data, facilitating a thorough evaluation of the device's performance and clinical outcomes.
As the field advances, understanding the nuances of these research frameworks will be crucial for clinical research directors aiming to navigate the complexities of post-market evaluations effectively, particularly within the context of Latin America.
Key Design Considerations for Post-Market Trials
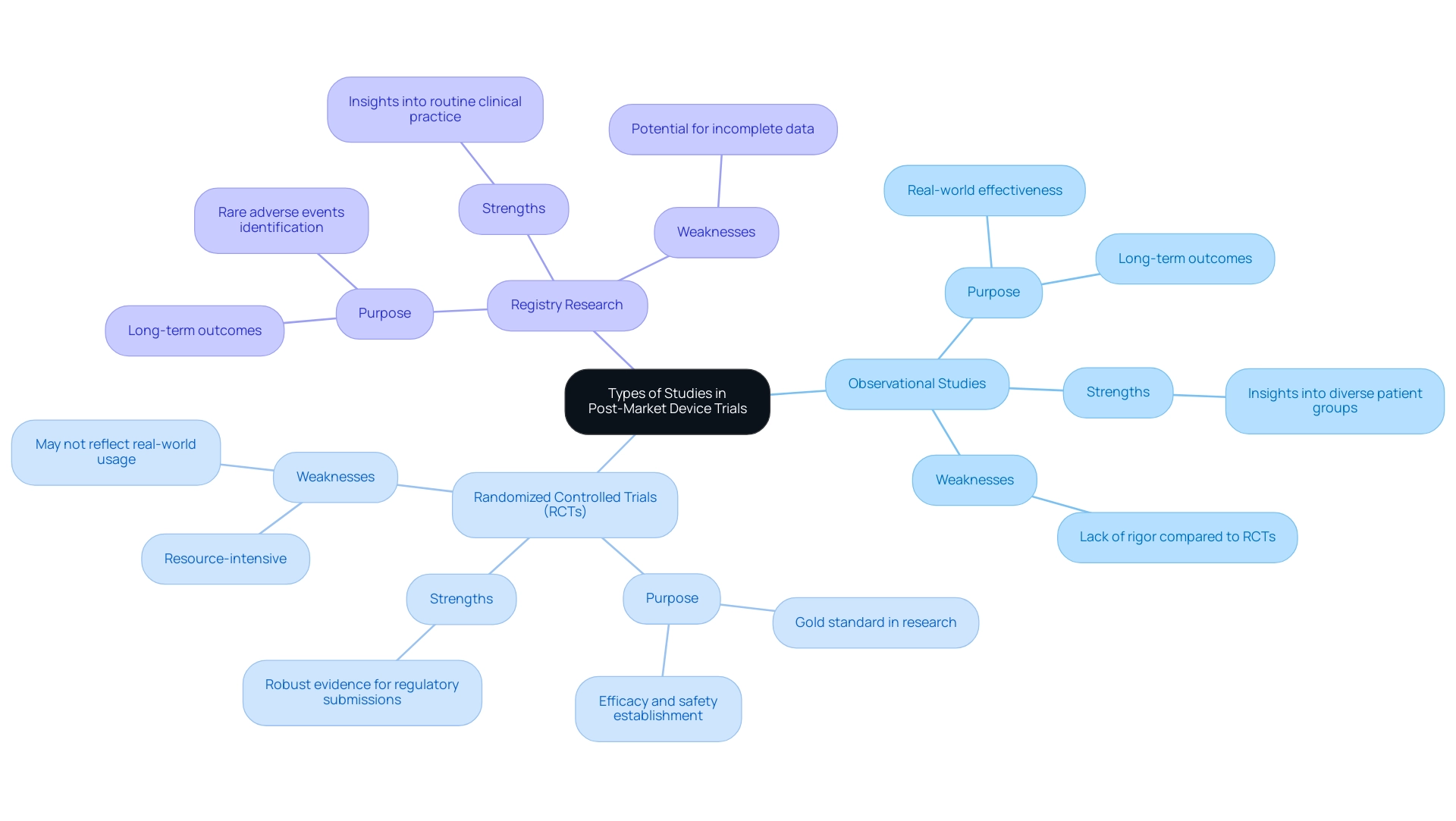
Creating follow-up evaluations requires careful consideration of several critical factors to ensure the success and reliability of research. Establishing clear objectives is paramount; these objectives guide the entire research process and aid in selecting appropriate endpoints that align with the study's aims. For example, in a trial for sitravatinib, the 120 mg dose was determined to be optimal based on trade-off desirability scores, highlighting the importance of sample size determination in post-market evaluations.
Recent research underscores that optimal sample sizes are vital for achieving reliable results and significantly affect the statistical power of outcomes. Moreover, the duration of follow-up must be adequate to collect meaningful data, particularly in medical instruments where long-term effects may be crucial. Researchers must also focus on the target population, ensuring it accurately represents the intended user base of the device. This consideration is essential in minimizing potential biases during data collection, which can skew results and lead to erroneous conclusions.
Incorporating these design elements not only bolsters the credibility of findings but also ensures that the research effectively addresses the posed questions. For instance, Agrawal et al. assessed receptor occupancy, pharmacokinetic parameters, and tumor shrinkage collectively for nivolumab dose selection, emphasizing the necessity of considering multiple factors in study design.
Furthermore, a recent analysis highlighted the importance of addressing missing data in preclinical research, examining various techniques such as multiple imputation and mixed models to improve outcome reliability. By documenting and tackling these challenges, researchers can better plan future studies and contribute to the overall advancement of medical devices. The HORIZON 3 study, which compared cediranib with bevacizumab in advanced metastatic colorectal cancer, exemplifies the implications of study design.
Although the study revealed no significant difference in progression-free survival between the two treatments, it illustrates the complexities inherent in developing effective clinical evaluations. Ultimately, the design of post-market device trials is crucial for generating meaningful data that can guide future device improvements and regulatory decisions, thereby supporting the ongoing evolution of the Medtech industry. With bioaccess®'s over 20 years of experience and comprehensive clinical trial management services—including expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—companies can adeptly navigate these complexities in Latin America, ensuring successful outcomes in their clinical research endeavors.
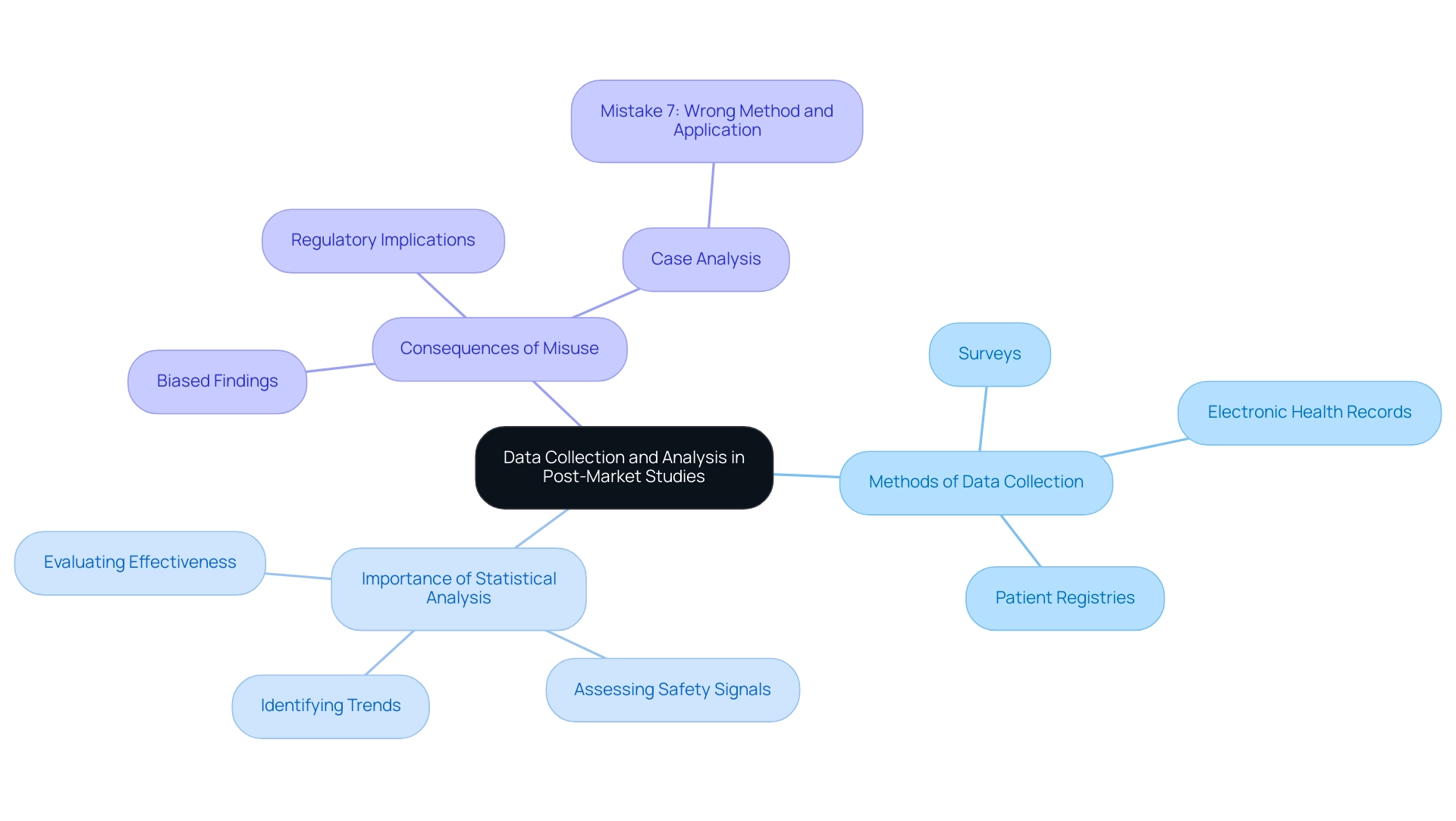
Data Collection and Analysis in Post-Market Studies
Data collection in post-market device trial design is multifaceted, employing methods such as surveys, electronic health records, and patient registries. It is paramount to ensure that the data gathered is relevant, accurate, and comprehensive. Statistical analysis serves as a cornerstone in interpreting this data, enabling researchers to identify trends, assess safety signals, and evaluate the device's effectiveness.
For instance, the risk of rhabdomyolysis with cerivastatin was found to be 5 to 10 times higher than that of other statins, illustrating the critical need for accurate data collection and analysis in assessing safety signals. Furthermore, non-compliance with best practices in clinical trials can lead to biased and misleading findings, compromising research integrity. This is evident in the case analysis titled "Mistake 7: Wrong method and wrong application of the methods," where manufacturers often misuse statistical techniques, applying them incorrectly to data that does not meet the necessary distribution or scale requirements.
Such misuse can lead to unreliable conclusions, such as incorrectly stating that there is no trend based on low confidence levels in the data. Therefore, employing robust statistical methods is essential for drawing valid conclusions, which can significantly influence regulatory actions and clinical practice. In 2025, the emphasis on accurate data collection methods and rigorous statistical analysis in post-market device trial design is more critical than ever, as regulatory bodies increasingly rely on these insights to ensure patient safety and device efficacy.
As outlined in EU regulations, a 'statistically significant' increase is defined in relation to the expected frequency or severity of incidents during a specified period, further emphasizing the importance of adhering to established protocols in clinical research. With over 20 years of experience in Medtech, bioaccess® provides comprehensive clinical study management services, including expertise in Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-market device trial design, ensuring the integrity of data collection and analysis. Additionally, our focus on Latin America and understanding of INVIMA's regulatory role enhance the reliability of clinical outcomes.
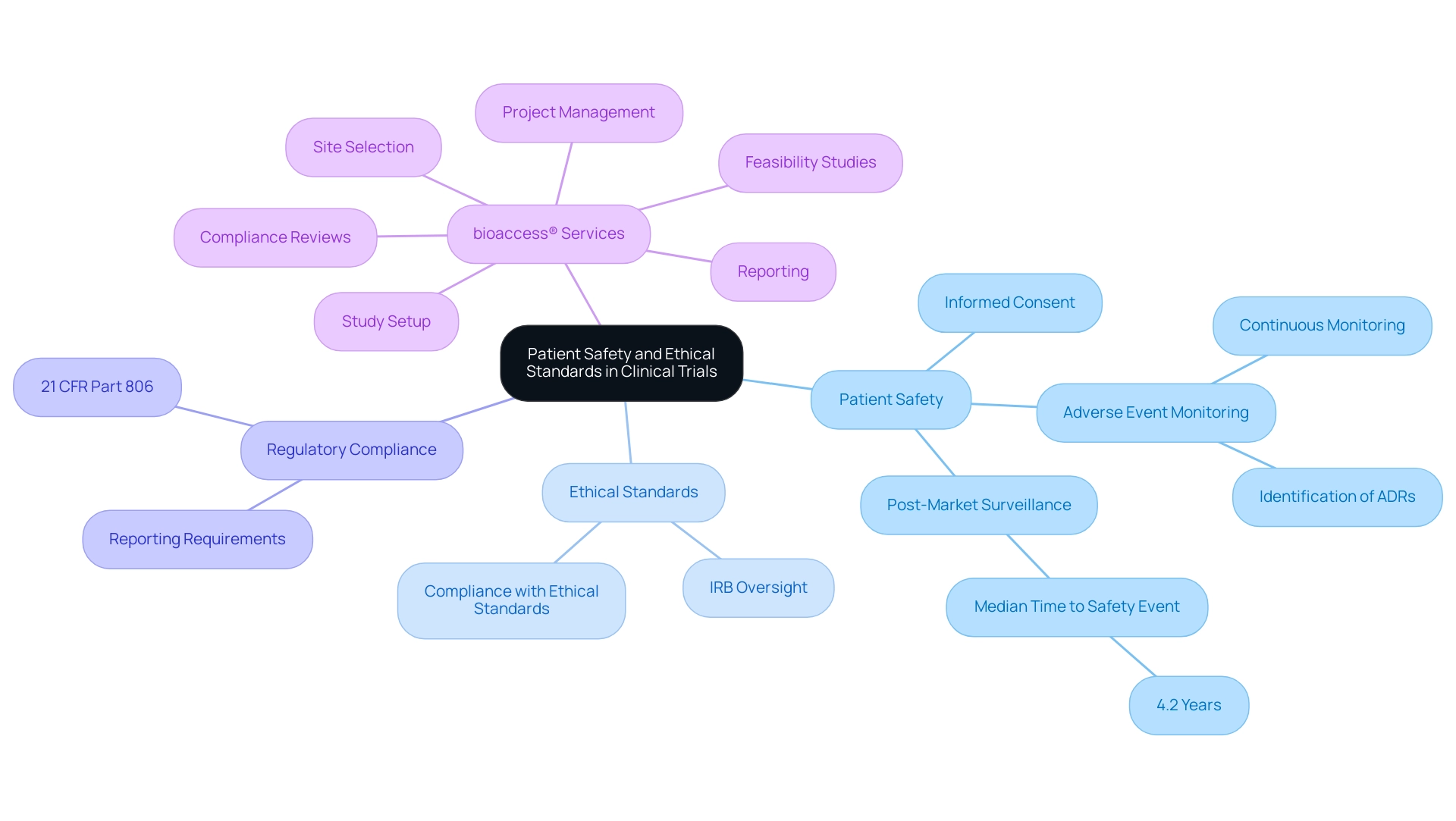
Ensuring Patient Safety and Ethical Standards in Trials
Patient safety remains the foremost priority in post-market device trial design, necessitating strict adherence to ethical standards. Researchers must obtain informed consent, ensuring that participants are fully aware of potential risks associated with the research. Continuous monitoring for adverse events is vital, allowing for prompt action to address any safety concerns that may arise during the trial.
Ethical oversight by Institutional Review Boards (IRBs) or ethics committees plays a crucial role in safeguarding the rights and welfare of participants throughout the research process. This oversight not only ensures compliance with established ethical standards but also fosters public trust and confidence in clinical research. As Yvonne Moores, Head of Operations at Quanticate, emphasizes, "Ethical standards are the backbone of clinical research, ensuring that participant welfare is prioritized at every phase of the project."
In the context of post-market surveillance, the identification of adverse drug reactions (ADRs) is particularly significant. A recent study monitoring the timing of post-market safety incidents revealed that the median duration from approval to the first safety event was 4.2 years, highlighting the need for continued vigilance even after a product has entered the market. This finding aligns with the regulatory reporting requirements outlined in 21 CFR Part 806, which mandates that manufacturers report corrections and removals to ensure patient safety.
Such findings emphasize the significance of strong patient safety protocols and ethical standards in clinical research, particularly regarding post-market device trial design.
Furthermore, bioaccess® provides comprehensive clinical study management services that include:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
With over 20 years of experience in Medtech, bioaccess® guarantees that all elements of the clinical study process are meticulously managed, improving the safety and effectiveness of medical products. This structured approach also includes navigating the regulatory compliance processes necessary for obtaining approvals from ethics committees and health ministries in Latin America.
Upholding these standards is essential for advancing medical technology while ensuring participant safety and maintaining public confidence in the research process.
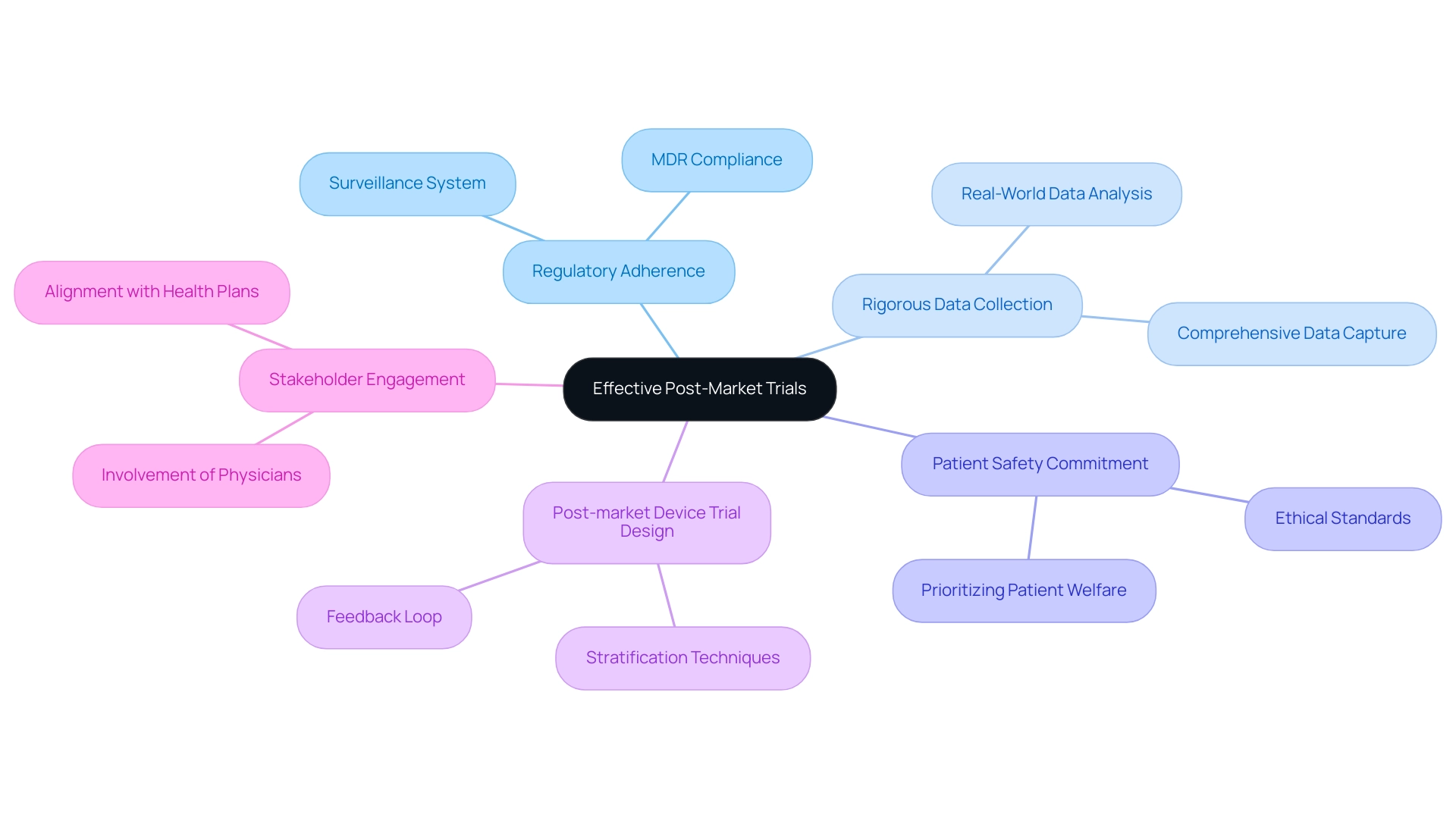
Summary of Essential Elements for Effective Post-Market Trials
Successful evaluations after market release are crucial in guaranteeing the continuous safety and effectiveness of medical products, a key aspect of post-market device trial design. These assessments must be based on a thorough comprehension of regulatory adherence, particularly under the Medical Device Regulation (MDR), which mandates that producers establish a surveillance system corresponding with the risks linked to their products. This compliance not only protects patient safety but also enhances the product's credibility in the market.
The impact of follow-up evaluations on medical device safety data cannot be overstated. By systematically collecting and analyzing real-world data, manufacturers can identify potential safety issues and address them proactively. Research indicates that non-randomized experiments may yield varying outcomes compared to randomized controlled experiments, underscoring the significance of design in capturing precise safety profiles.
A significant case study demonstrating the importance of efficient market follow-up studies is the ongoing interest in comparative effectiveness research. This initiative, recognized by the U.S. government through the Agency for Healthcare Research and Quality (AHRQ), highlights the necessity for direct comparisons of medical devices to ensure optimal treatment options and cost-effectiveness, particularly concerning Medicare Part D. As S Schneeweiss from the Division of Pharmacoepidemiology and Pharmacoeconomics at Brigham and Women’s Hospital states, 'In the absence of sufficient direct effectiveness studies, comparative effectiveness research attempts to address the problem of limited generalizability to routine care and the lack of an active comparison group by analyzing post-marketing drug use data.'
Key elements for successful post-market trials include:
- Rigorous Data Collection: Ensuring comprehensive data capture to facilitate robust analysis.
- Patient Safety Commitment: Upholding ethical standards and prioritizing patient welfare throughout the post-market device trial design process.
- Post-market Device Trial Design: Utilizing stratification techniques to estimate treatment effects across diverse patient subgroups, thereby enhancing the relevance of findings. This approach allows for treatment effect estimates without discarding non-adherent patients, providing a comprehensive analysis.
- Stakeholder Engagement: Involving physicians and health plans to align trial objectives with real-world clinical needs.
- Post-market Device Trial Design: Implementing a feedback loop for the continuous monitoring and ongoing assessment of performance after launch.
By concentrating on these essential elements, medical equipment manufacturers can not only meet regulatory expectations but also significantly enhance patient outcomes. The commitment to ethical standards and patient safety fosters public confidence in medical technologies, ultimately leading to better healthcare outcomes and a more robust market presence. With over 20 years of experience in Medtech, bioaccess® offers the expertise and customized approach needed to navigate the complexities of clinical trials in Latin America effectively, ensuring compliance and enhancing the safety and efficacy of their devices.
Conclusion
Post-market trials are essential for guaranteeing the ongoing safety and effectiveness of medical devices, serving as a crucial link between regulatory compliance and real-world application. These studies yield invaluable insights by gathering real-world data that aids in identifying unforeseen issues, evaluating long-term performance, and informing future regulatory decisions. With the forthcoming Medical Device Regulation (MDR) in 2025, manufacturers must adeptly navigate a complex landscape of regulatory requirements to sustain compliance and promote innovation.
The various types of studies—including observational studies, randomized controlled trials (RCTs), and registry studies—each fulfill a distinctive role in assessing device performance post-market. The significance of well-designed trials cannot be overstated; clear objectives, adequate follow-up durations, and precise target populations are vital for generating reliable data. Furthermore, robust data collection and analysis methods are critical for identifying safety signals and ensuring the integrity of research findings.
Safety and ethical considerations remain paramount throughout the trial process. Ensuring informed consent, continuous monitoring for adverse events, and adhering to ethical standards not only safeguard participants but also bolster public trust in medical technologies. By prioritizing these elements, manufacturers can markedly improve patient outcomes and sustain their market presence.
In conclusion, effective post-market trials are indispensable for the ongoing evolution of the Medtech industry. By concentrating on regulatory compliance, rigorous data collection, and patient safety, manufacturers can adeptly navigate the complexities of clinical trials. The commitment to ethical practices and continuous monitoring reinforces the integrity of medical devices and fosters public confidence, ultimately leading to enhanced healthcare outcomes for patients worldwide.
Frequently Asked Questions
What is the purpose of post-market studies for medical devices?
Post-market studies are designed to ensure ongoing patient safety, evaluate long-term performance, and gather real-world data to uncover unforeseen issues that may arise during routine use of medical devices.
What are the regulatory requirements for post-market studies as of 2025?
As of 2025, the Medical Device Regulation (MDR) requires stringent monitoring after market release to maintain CE marking for devices, ensuring compliance that protects patient safety and encourages business growth and innovation.
What are the potential consequences for manufacturers who neglect post-market surveillance (PMS) regulations?
Manufacturers that neglect PMS regulations may face severe repercussions, including regulatory non-compliance, fines, and product recalls, highlighting the necessity of adherence to these regulations.
Why is post-marketing research important in evaluating medical devices?
Post-marketing research is crucial for assessing the relevance of premarketing data in real-world settings, as it helps identify differences in device performance across various patient demographics.
What role does statistical methodology play in post-market evaluations?
Statistical considerations, including sample size and research design, are essential in follow-up evaluations to address the unique objectives and challenges of each phase of clinical evaluations.
How does bioaccess® support manufacturers in post-market studies?
Bioaccess® manages post-market clinical follow-up research and offers services such as feasibility studies, investigator selection, regulatory compliance, and project management to help manufacturers navigate post-market trial complexities.
What is the FDA's role in post-market surveillance?
The FDA mandates that manufacturers conduct evaluations after market release to assess the safety and effectiveness of medical devices, requiring timely reporting of adverse events and development of comprehensive PMS plans.
What initiatives has the FDA implemented to enhance post-market surveillance?
The FDA has established an automated tracking system to improve oversight of ongoing surveillance efforts, ensuring that manufacturers meet their monitoring obligations effectively.
How does bioaccess® ensure compliance and client trust in its processes?
Bioaccess® is committed to information security and client trust, addressing concerns through a dedicated Grievance and Data Protection Officer to foster transparency and compliance in all operations.

