Overview
The article focuses on identifying essential recruitment strategies for medical device trials in Latin America, emphasizing the importance of understanding local regulations, cultural contexts, and technological advancements. It supports this by detailing various approaches, such as forming partnerships with local healthcare providers, utilizing digital platforms for outreach, and adapting strategies to align with regional demographics, which collectively enhance participant engagement and improve recruitment outcomes.
Introduction
The regulatory environment for medical device trials in Latin America is a complex tapestry, woven from the diverse regulations and requirements of each country. With regulatory bodies such as ANVISA in Brazil and COFEPRIS in Mexico at the forefront, understanding these frameworks is paramount for the success of clinical trials.
As companies strive to navigate this intricate landscape, the importance of tailored patient recruitment strategies becomes increasingly evident. Engaging local regulatory consultants and healthcare providers can provide essential insights, ensuring compliance and fostering trust among potential participants.
This article delves into the multifaceted approaches necessary for optimizing recruitment in Latin America, highlighting the significance of:
- Cultural considerations
- Technological advancements
- Collaborative partnerships
in enhancing trial outcomes. By exploring these critical elements, stakeholders can better position themselves for success in this dynamic region.
Navigating the Regulatory Landscape for Medical Device Trials in Latin America
Navigating the regulatory landscape for medical device studies in Latin America presents unique challenges that vary significantly from one country to another. Each country implements its own set of rules, with agencies such as ANVISA in Brazil and COFEPRIS in Mexico playing crucial roles in the supervision of research studies. Notably, regulatory reliance pathways are officially presented in countries such as Argentina, Chile, Colombia, and others, illustrating the diverse frameworks in place.
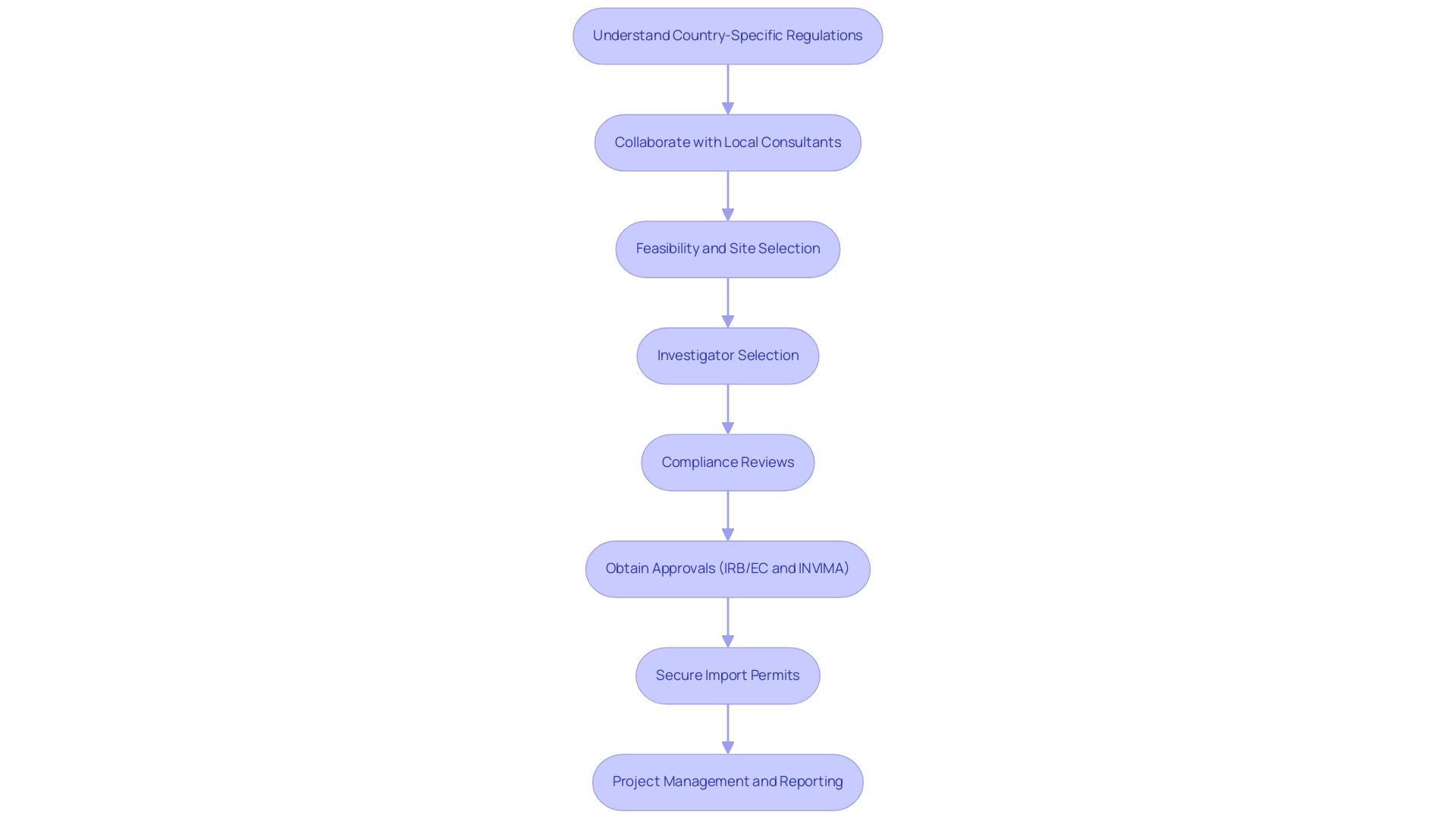
To develop effective Recruitment Strategies for Medical Device Trials in Latin America, it is crucial to possess a robust understanding of these regulations to ensure compliance and prevent potential delays. Collaborating with local regulatory consultants can offer invaluable insights into the specific requirements and nuances of clinical studies in each country, ensuring that all protocols are adhered to. Our service capabilities encompass:
- Feasibility and selection of research sites
- Investigator selection
- Compliance reviews
- Setup (including IRB/EC approval and INVIMA approval)
- Import permits
- Project management
- Reporting
This comprehensive approach not only streamlines the approval process but also enhances the likelihood of successful participant recruitment, which is essential in recruitment strategies for medical device trials in Latin America. Moreover, Colombia provides competitive benefits for first-in-human studies, such as cost efficiency, regulatory speed, high-quality healthcare, and R&D tax incentives. As Adrian Ebner, a notable individual in medical research, stresses, "Understanding these dynamics is essential for any study aimed at achieving regulatory compliance and operational success."
Moreover, the restricted uptake of ICH guidelines in LATAM highlights the difficulties of regulatory alignment and its effect on the quality and efficiency of studies in the region. We encourage you to engage with our services to navigate these complexities effectively.
Tailoring Recruitment Strategies to Local Demographics and Culture
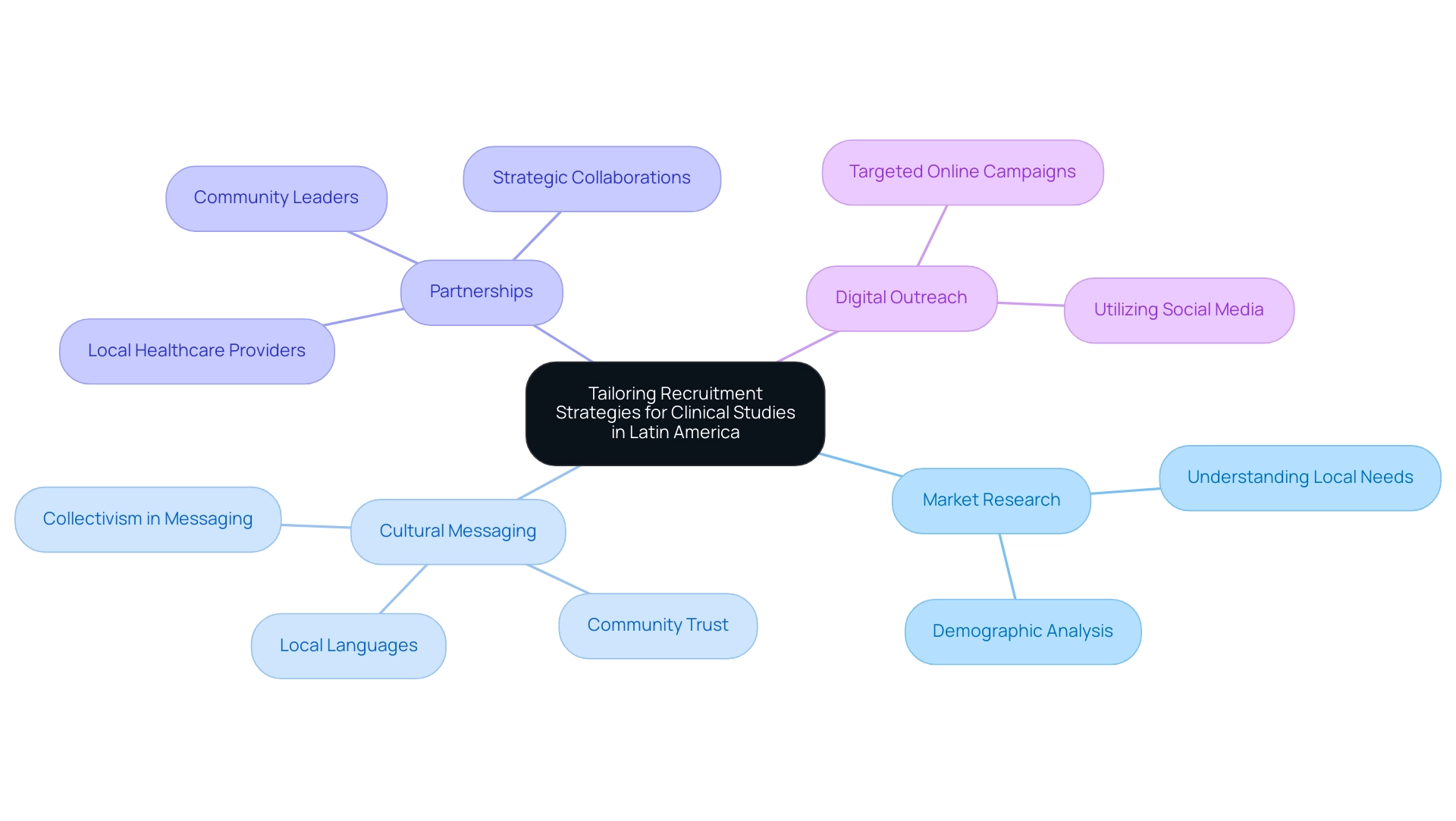
To enhance patient enrollment for clinical studies in Latin America, it is essential to implement Recruitment Strategies for Medical Device Trials in Latin America that are customized to represent the region's rich diversity in demographics and cultural contexts. Conducting thorough market research is essential to grasp the unique needs, preferences, and concerns of potential participants across various locales. For instance, the use of local languages and culturally relevant messaging can significantly enhance engagement and hiring efforts.
Moreover, forming alliances with local healthcare providers and community leaders not only cultivates trust but also boosts credibility, encouraging wider involvement in studies. A notable case study titled "Motivators for Clinical Trial Participation Among Latinas" found that altruism, particularly the desire to help family members, was a strong motivator for participation, highlighting the importance of collectivism in Latino culture. Moreover, recent discoveries suggest that marital status and language acculturation are important indicators of willingness to engage in research studies, highlighting the necessity for recruitment strategies for medical device trials in Latin America that take these elements into account.
The partnership between bioaccess™ and Caribbean Health Group, revealed on March 29, 2019, at PROCOLOMBIA's office in Miami, seeks to establish Barranquilla as a prominent location for medical studies in Latin America, backed by Colombia's Minister of Health. This initiative enhances the region’s appeal for potential study participants. Digital platforms widely used in specific countries should be leveraged to reach a larger audience, facilitating connections with potential participants.
Such culturally aware methods are crucial in boosting enrollment rates and are essential components of effective recruitment strategies for medical device trials in Latin America, ensuring that studies are representative of the populations they intend to assist, ultimately contributing to more precise medicine treatments for cancer patients. Furthermore, GlobalCare Clinical Trials' partnership with bioaccess™ has achieved over a 50% reduction in participant acquisition time and a remarkable 95% retention rate, demonstrating the effectiveness of these strategic collaborations. The expanding media attention on research studies in Latin America by Clinical Leader also indicates the rising emphasis and funding in this sector, which can beneficially influence local economies through job creation and enhancements in healthcare.
Comprehensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project oversight
- Reporting
are integral to enhancing participant strategies and ensuring successful study outcomes.
Leveraging Technology for Efficient Recruitment
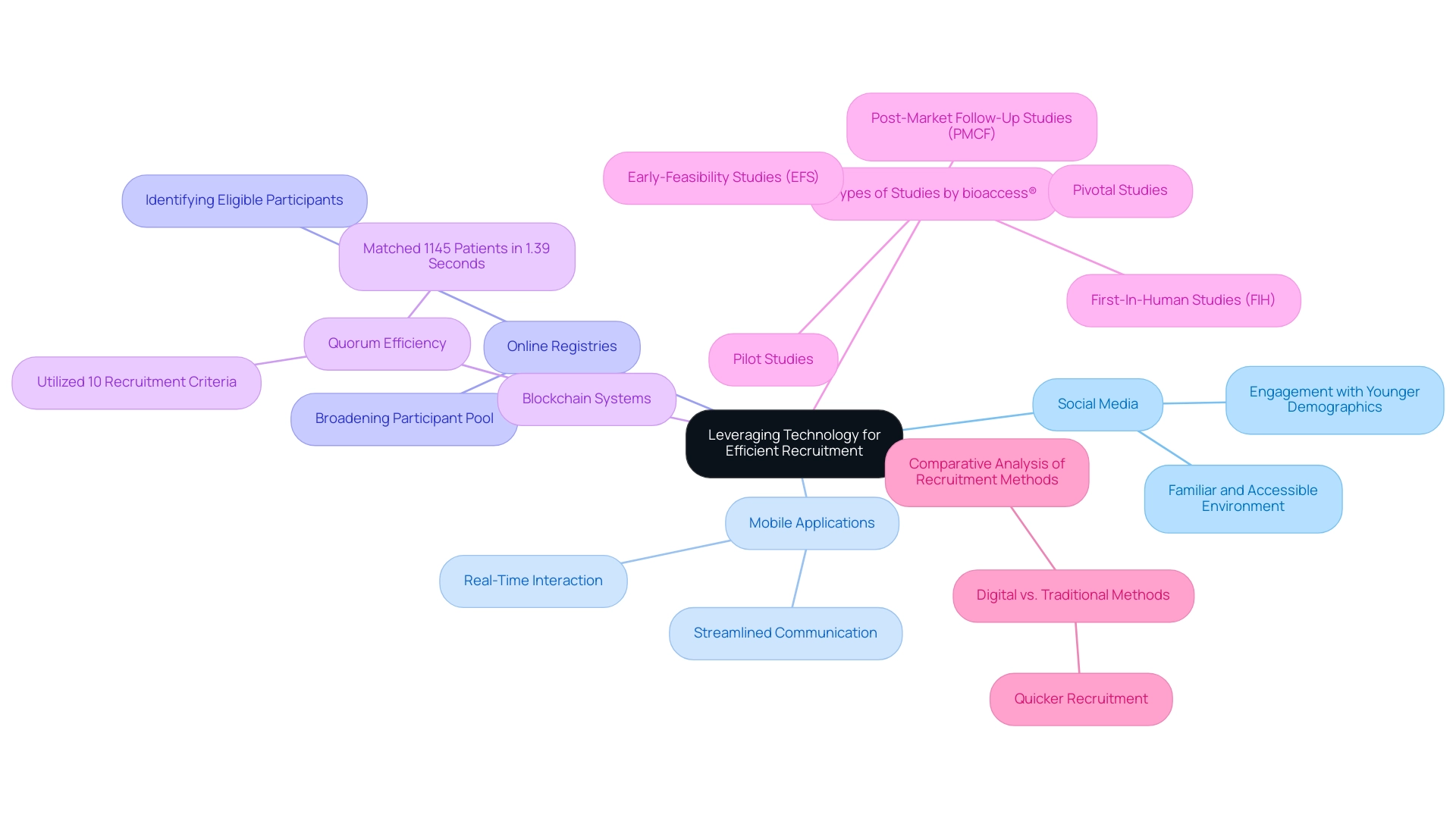
The incorporation of technology into participant engagement strategies is transforming recruitment strategies for medical device trials in Latin America. Social media platforms stand out as powerful tools for reaching younger demographics, enabling researchers to engage with potential participants in a familiar and accessible environment. Mobile applications further enhance this engagement by allowing for real-time interaction and streamlined communication.
Furthermore, online registries are essential for implementing recruitment strategies for medical device trials in Latin America, enabling clinical research groups to effectively identify and engage with individuals who fulfill specific trial criteria, thereby broadening their participant pool. A comparative analysis of strategies for stroke survivors, titled 'Comparative Analysis of Strategies for Stroke Survivors,' revealed that digital approaches significantly outperformed traditional methods, resulting in quicker participant enrollment. Furthermore, a blockchain system named Quorum has shown exceptional efficiency, matching 1145 patients in only 1.39 seconds using 10 selection criteria.
As Florence Mowlem, PhD, Vice President of Science for ObvioHealth, stated, 'I hope this can be a turning point for the industry with regard to comparability testing. We can stop having [comparability] conversations so frequently, and instead we can start talking about optimizing our electronic measures for all individuals.' By utilizing these technological advancements along with the comprehensive study management services provided by bioaccess®, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
research teams can enhance their participant selection processes, which are essential for effective Recruitment Strategies for Medical Device Trials in Latin America.
'bioaccess®'s tailored strategy guarantees adaptability and corresponds with the desires of contemporary participants, ultimately enhancing the effectiveness of medical studies.
Building Partnerships with Local Healthcare Providers
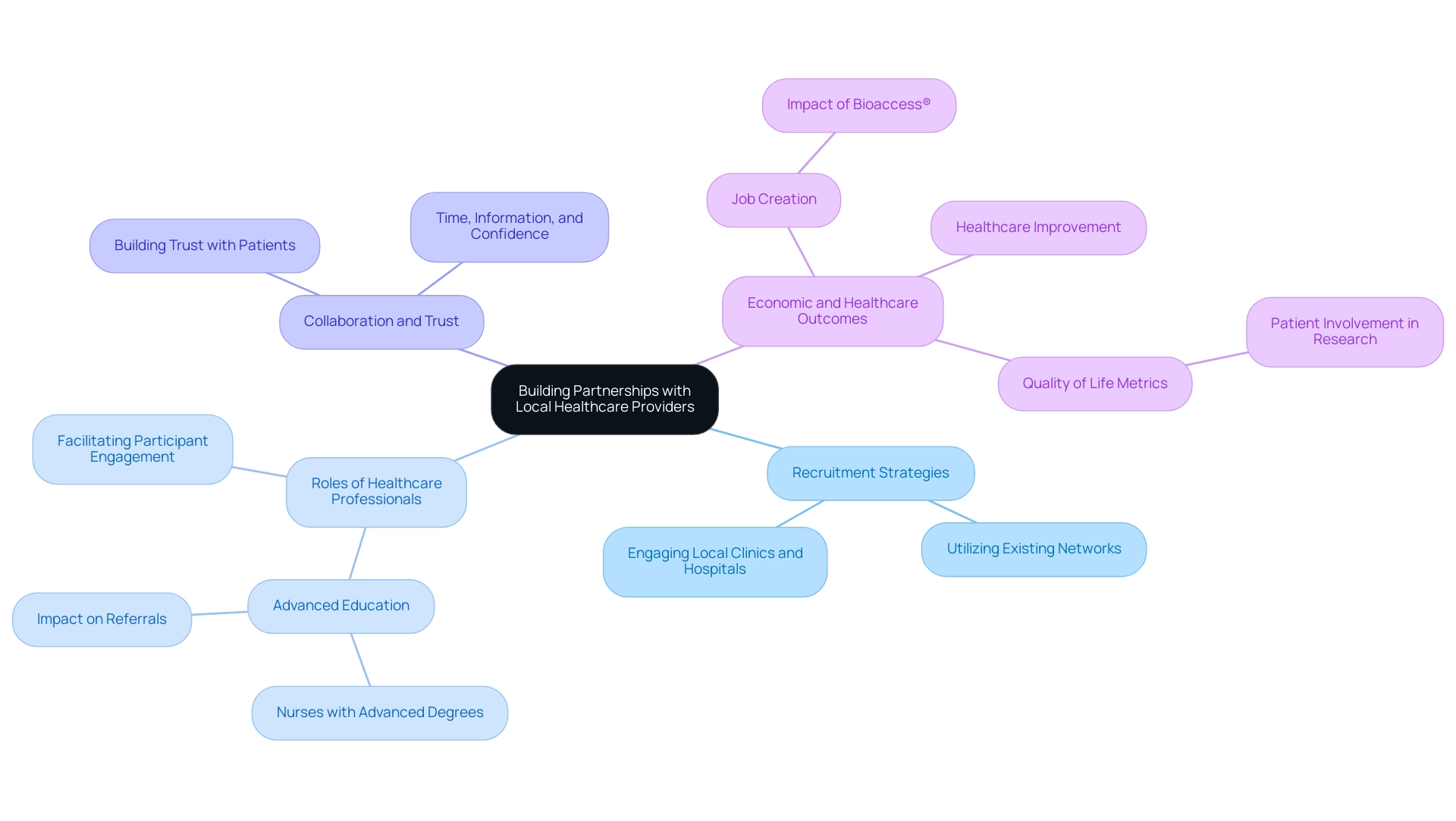
Establishing partnerships with local healthcare providers is a pivotal strategy for implementing effective recruitment strategies for medical device trials in Latin America. Healthcare experts, especially individuals with higher education, frequently have well-developed connections with possible participants, allowing them to efficiently inform individuals about the benefits and importance of research studies. Research indicates that nurses with advanced degrees are significantly more likely to refer individuals compared to registered nurses, underscoring the value of engaging these professionals.
Additionally, a case study titled 'Misconceptions About Health Care Providers' Interest in Clinical Research' revealed that healthcare providers can facilitate participant engagement in clinical studies if they possess sufficient time, information, and confidence, which aligns with the theme of collaboration and trust. Local providers play a crucial role in recognizing suitable candidates from their client groups, thus simplifying the hiring process. By partnering with local clinics and hospitals, researchers can utilize existing networks, fostering a foundation of trust and collaboration that significantly improves patient willingness to engage in studies.
In Latin America, recent studies have demonstrated that this approach not only aids recruitment but also supports recruitment strategies for medical device trials in Latin America, as healthcare providers become more confident and informed advocates for trial participation. Additionally, with bioaccess®'s expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), the impact of these studies extends to job creation, economic growth, and healthcare improvement in local economies. For example, bioaccess® has contributed to the creation of over 500 jobs in the region through its research studies, highlighting the tangible benefits of these partnerships.
As highlighted by the European Medicines Agency (EMA), comprehending health-related quality of life metrics is essential in assessing medicinal products, further stressing the significance of patient involvement in research studies.
Implementing Community Engagement Initiatives
Community engagement initiatives play a crucial role in enhancing Recruitment Strategies for Medical Device Trials in Latin America, aligning with comprehensive research management services that encompass:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
By organizing health fairs, informational sessions, and workshops, we provide effective platforms to educate the community about research and its potential benefits, which supports our recruitment strategies for medical device trials in Latin America and addresses the challenges faced by Medtech companies. These initiatives not only spread essential information but also address common issues and misunderstandings related to research studies.
Actively involving community leaders enhances the credibility of these initiatives, fostering trust among potential participants. As emphasized by Mike Hennessy Jr., 'This November edition of ACT examines those technological advancements in trial recruitment and data collection and management that are aimed at empowering the research community's most valuable asset—the individual.' Understanding factors that influence individual decision-making, such as accessibility and personal preferences, is key to improving engagement.
Insights from the case study titled 'Factors Affecting Patient Decision-Making in Trials' reveal that recognizing and addressing these factors is essential for improving patient engagement and participation in research. Additionally, health fairs have demonstrated their effectiveness in attracting participants for studies, enhancing awareness and interest in ongoing research initiatives. Significantly, Neighborhood Healthcare has been performing medical research for 20 years, highlighting the experience and credibility of health centers in establishing research partnerships.
By fostering an open conversation and establishing supportive settings, researchers can effectively motivate individuals to consider involvement in research studies, ultimately advancing biomedical knowledge and promoting fair health outcomes. Additionally, these community engagement efforts contribute to local economic growth by creating jobs and fostering international collaboration in the region, which is essential for driving innovation and improving health outcomes.
Utilizing Patient Advocacy Groups
Working alongside advocacy organizations (PAGs) serves as a crucial approach for improving enrollment in clinical trials, which aligns with effective recruitment strategies for medical device trials in Latin America. These organizations possess well-established networks that facilitate connections with specific patient populations. Through this collaboration, researchers can gain invaluable insights into the needs and concerns of potential participants, which will enhance their recruitment strategies for medical device trials in Latin America.
As highlighted by a Principal Investigator, 'The quality, quantity, efficiency, and pace of clinical research in rare diseases is greatly enhanced by the types of investigator-patient partnerships that have developed within the RDCRN.' Advocacy groups also play a critical role in raising awareness about ongoing studies, which is essential for effective recruitment strategies for medical device trials in Latin America, significantly improving outreach efforts and ultimately increasing participation rates. Notably, attending Consortium investigator meetings has a mean impact rating of 4.8, underscoring the importance of these gatherings in developing recruitment strategies for medical device trials in Latin America.
Additionally, collaborations such as that of bioaccess™ and the Caribbean Health Group, backed by Colombia's Minister of Health, are vital in establishing Barranquilla as a prominent location for implementing Recruitment Strategies for Medical Device Trials in Latin America. The Minister's backing reinforces the initiative's credibility and potential for success. The success of the Rare Disease Clinical Research Network (RDCRN) in involving participants for the development of research networks serves as a valuable model for other research initiatives.
Their method, which highlights the engagement of PAGs in study design, participant selection, and sharing of outcomes, illustrates the significant effect that patient advocacy can have on recruitment strategies for medical device trials in Latin America, relevant to both rare and prevalent disorders. These collaborative efforts not only enhance hiring efficiency—evidenced by GlobalCare Clinical Trials partnering with bioaccess™ to achieve over a 50% reduction in hiring time and 95% retention rates—but also drive economic growth and healthcare improvement in local communities. Furthermore, bioaccess™ and Caribbean Health Group provide extensive service capabilities, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
All of which contribute to the overall success of research studies in the region.
Monitoring and Adapting Recruitment Strategies
Effective monitoring and adaptation of recruitment strategies for medical device trials in Latin America are essential for optimizing performance throughout the clinical trial process. Establishing key performance indicators (KPIs) is crucial for evaluating hiring success. By systematically analyzing data on participant demographics, response rates, and engagement levels, research teams can pinpoint areas needing improvement and make necessary adjustments.
For instance, using warm transfer techniques can expedite scheduling by up to 40%, demonstrating how refined processes can lead to better outcomes. Companies like Clariness set ambitious targets for contacting patients—aiming for less than 20 minutes for first contact and 10 days for scheduling visits. This focus on timely engagement not only enhances site performance but also significantly increases patient consent numbers.
Moreover, with bioaccess®'s extensive expertise in comprehensive clinical study management services—including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
organizations can ensure that participant strategies are continuously optimized. The inclusion of compliance reviews and reporting processes, such as study status and adverse event reporting, is critical to maintaining regulatory standards and enhancing study efficiency. By tracking site engagement with referrals, organizations can determine if extra assistance is required for underperforming sites, thus ensuring the implementation of effective recruitment strategies for medical device trials in Latin America.
As Cloudbyz aptly states, "We strive to be a trusted partner, dedicated to providing exceptional service, exceptional products, and unparalleled support." This proactive approach to recruitment strategies for medical device trials in Latin America, combined with an understanding of historic trends and the adoption of eSignatures, ensures that strategies remain aligned with the evolving needs of the target population, fostering better engagement and more successful trial outcomes.
Conclusion
Navigating the regulatory landscape for medical device trials in Latin America requires a nuanced understanding of diverse regulations and cultural dynamics. With the involvement of key regulatory bodies such as ANVISA in Brazil and COFEPRIS in Mexico, stakeholders must prioritize compliance and adopt tailored patient recruitment strategies. Engaging local regulatory consultants and healthcare providers not only fosters trust but also enhances the likelihood of successful participant recruitment.
Cultural considerations, technological advancements, and collaborative partnerships emerge as critical components in optimizing recruitment efforts. Tailoring recruitment strategies to local demographics and leveraging technology can significantly improve engagement rates, while establishing partnerships with local healthcare providers and patient advocacy groups facilitates trust and enhances outreach. Implementing community engagement initiatives further educates potential participants, addressing common misconceptions and promoting clinical trial participation.
Ultimately, the integration of these multifaceted approaches is essential for enhancing trial outcomes in Latin America. By focusing on culturally informed strategies and utilizing technology to streamline processes, stakeholders can better position themselves for success in this dynamic region. Emphasizing collaboration and continuous monitoring of recruitment strategies will not only improve participant engagement but also drive economic growth and healthcare improvements within local communities.
Frequently Asked Questions
What are the main regulatory challenges for medical device studies in Latin America?
The regulatory landscape for medical device studies in Latin America varies significantly by country, with each implementing its own set of rules. Agencies such as ANVISA in Brazil and COFEPRIS in Mexico play crucial roles in supervising research studies.
How can understanding regulations impact recruitment strategies for medical device trials?
A robust understanding of regulations is crucial to ensure compliance and prevent delays in recruitment strategies for medical device trials. Collaborating with local regulatory consultants can provide insights into specific requirements and nuances of clinical studies in each country.
What services are offered to facilitate medical device trials in Latin America?
Services include feasibility and selection of research sites, investigator selection, compliance reviews, setup (including IRB/EC approval and INVIMA approval), import permits, project management, and reporting.
What advantages does Colombia offer for first-in-human studies?
Colombia provides benefits such as cost efficiency, regulatory speed, high-quality healthcare, and R&D tax incentives, making it an attractive location for first-in-human studies.
How does cultural context influence participant recruitment in Latin America?
Customized recruitment strategies that reflect the region's diversity in demographics and cultural contexts are essential. Utilizing local languages, culturally relevant messaging, and forming alliances with local healthcare providers can enhance engagement and trust.
What factors motivate participation in clinical trials among Latinas?
Altruism, particularly the desire to help family members, is a strong motivator for participation, emphasizing the importance of collectivism in Latino culture. Marital status and language acculturation also influence willingness to engage in research studies.
What impact do strategic partnerships have on participant recruitment?
Strategic partnerships, such as that between bioaccess™ and Caribbean Health Group, can enhance the appeal of regions for medical studies and improve participant recruitment efficiency, as demonstrated by significant reductions in acquisition time and high retention rates.
What comprehensive management services are integral to successful study outcomes?
Comprehensive management services include feasibility assessments, site selection, compliance reviews, setup, import permits, project oversight, and reporting, all of which enhance participant strategies and contribute to successful study outcomes.




