Overview
The article outlines seven key benefits and challenges of remote monitoring in clinical trials, focusing on its potential for cost efficiency, improved patient recruitment, enhanced patient care, interoperability issues, and better oversight and risk management. These points are supported by various studies and statistics that demonstrate how remote monitoring can reduce expenses, increase participant engagement, and facilitate timely interventions, while also addressing the need for interoperable technologies to optimize data sharing and communication.
Introduction
In the evolving landscape of clinical research, remote monitoring emerges as a transformative force, reshaping traditional methodologies and enhancing efficiency across various dimensions. By leveraging advanced digital technologies, clinical trials can significantly reduce costs, improve patient recruitment, and optimize care delivery, all while navigating the complexities of interoperability and risk management.
This article delves into the multifaceted benefits of remote monitoring, highlighting its potential to streamline operations, enhance patient engagement, and ultimately drive better outcomes in clinical trials. As organizations increasingly adopt these innovative solutions, understanding the implications and advantages of remote monitoring becomes essential for stakeholders aiming to stay competitive in this dynamic field.
Cost Efficiency: How Remote Monitoring Reduces Clinical Trial Expenses
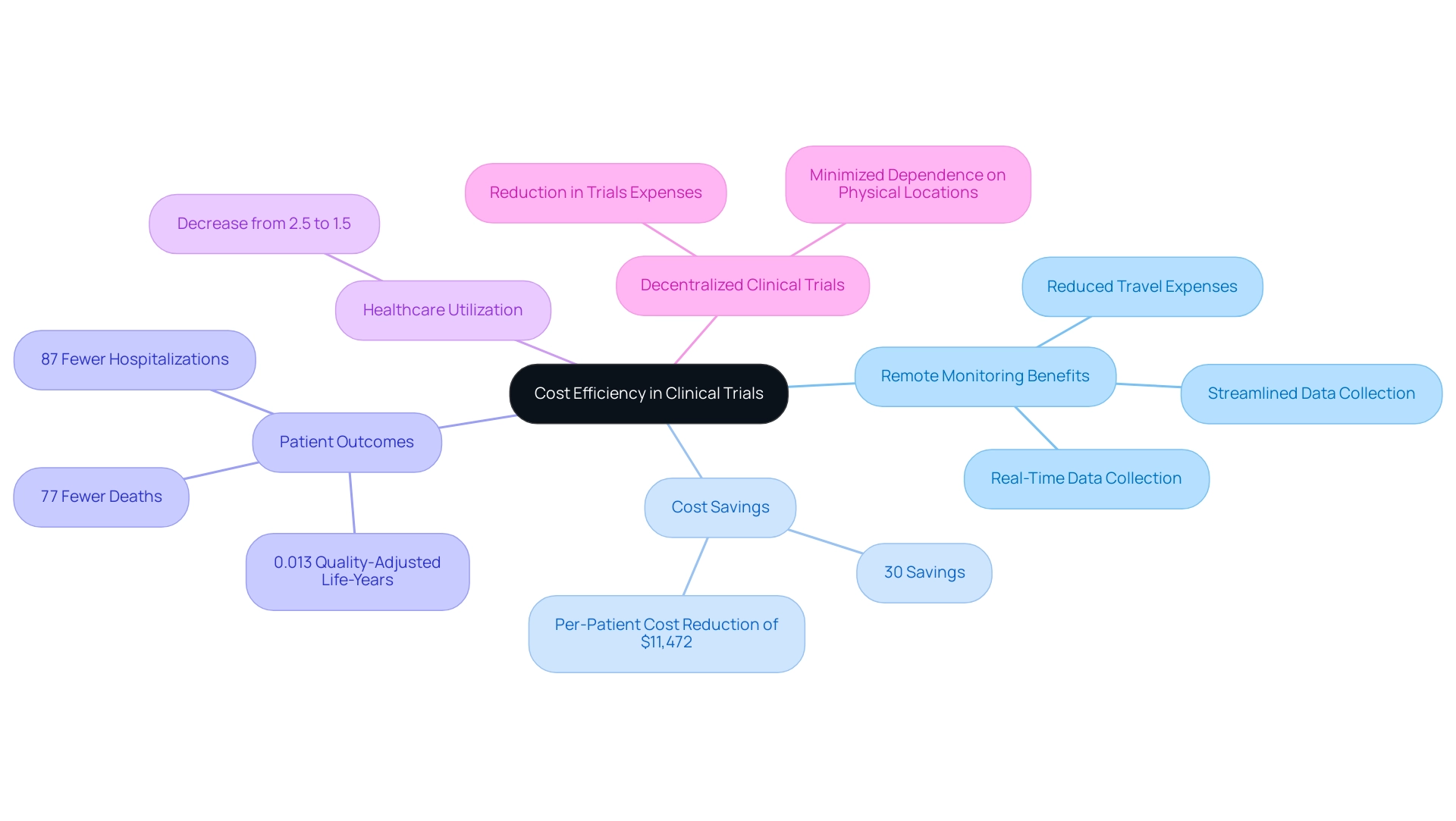
The concept of Remote Monitoring in Clinical Trials: Benefits and Challenges presents a considerable opportunity for reducing expenses compared to traditional evaluation techniques. By utilizing digital tools, sponsors can effectively minimize travel expenses associated with site visits and reduce the necessity for on-site staff, highlighting the Remote Monitoring in Clinical Trials: Benefits and Challenges while streamlining data collection processes. For instance, recent advancements in distance patient observation technologies enable real-time data collection, significantly reducing the time dedicated to data entry and analysis.
Moreover, the decreased overhead costs associated with physical sites and the ability to simultaneously monitor multiple locations contribute to enhanced cost efficiency. Research suggests that the implementation of remote monitoring in clinical trials: benefits and challenges can produce savings of up to 30% during particular trial stages. Furthermore, a recent study in the Journal of American Medicine Association disclosed that distant oversight resulted in 87% fewer hospitalizations and 77% fewer fatalities, along with a per-patient cost decrease of $11,472 compared to standard care, alongside improvements of 0.013 quality-adjusted life-years.
Significantly, the rate of healthcare usage declined from 2.5 to 1.5 following the intervention, demonstrating the substantial effect of distant oversight. Additionally, off-site assessments evaluate safety and efficacy while gathering continuous data via applications, strengthening the effectiveness of these technologies. A case study emphasizes that incorporating clinical study software can reduce overall clinical study expenses by around 30% through decentralized clinical study models, which minimize dependence on physical locations and in-person visits.
Such metrics demonstrate not only the financial advantages but also the potential for enhanced outcomes, highlighting Remote Monitoring in Clinical Trials: Benefits and Challenges as an appealing choice for organizations concentrated on budgetary efficiency in clinical research.
Enhancing Patient Recruitment and Engagement Through Remote Monitoring
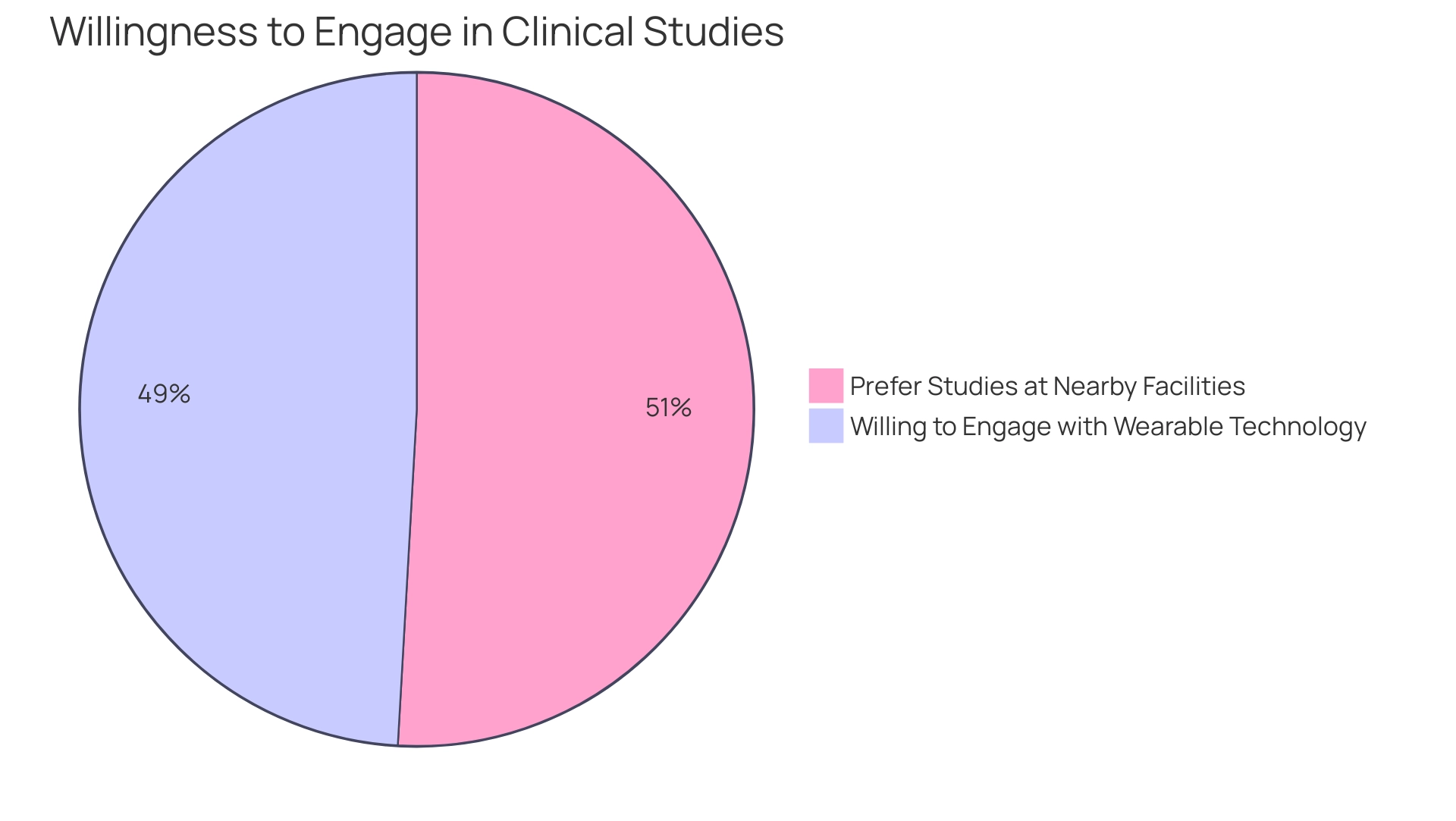
Remote monitoring greatly improves participant recruitment by expanding access to clinical studies for individuals in rural regions or those experiencing mobility difficulties. The adoption of technologies such as mobile applications and wearable devices allows participants to partake in studies from the comfort of their own homes, which has been shown to improve enrollment rates. A recent survey emphasized this trend, showing that:
- 82% of individuals with cancer showed a willingness to engage in studies that employed wearable technology.
- 85% stated they would be more inclined to join studies held at nearby facilities.
This suggests that local engagement and technology can significantly improve recruitment efforts. Furthermore, distant observation promotes ongoing participant involvement through frequent check-ins and feedback mechanisms, which are essential for maintaining interest and compliance with study protocols. Research shows that studies using distant observation report increased retention rates, as patients feel more engaged and supported during their involvement.
According to Morgan et al., innovative online strategies have effectively recruited diverse samples for randomized controlled studies (RCTs), highlighting the role of Remote Monitoring in Clinical Trials: Benefits and Challenges in enhancing inclusivity and engagement in clinical research. Additionally, the case study on diversity in clinical research recruitment highlights that minorities and people of color are historically underrepresented, with logistical concerns identified as a primary barrier to participation. Utilizing technology to reduce site visits may improve enrollment, especially among diverse communities, suggesting that observational trials could serve as a gateway for broader research participation.
Adding Value to Patient Care: The Benefits of Remote Monitoring
The concept of Remote Monitoring in Clinical Trials: Benefits and Challenges significantly enhances care by facilitating continuous health observation and enabling timely interventions. Wearable devices, for example, can monitor vital signs and alert healthcare providers of any anomalies, enabling swift action if an individual's condition deteriorates. This proactive approach not only enhances safety but also promotes better adherence to treatment protocols; individuals receive tailored feedback and support through these devices.
Significantly, a recent study disclosed that:
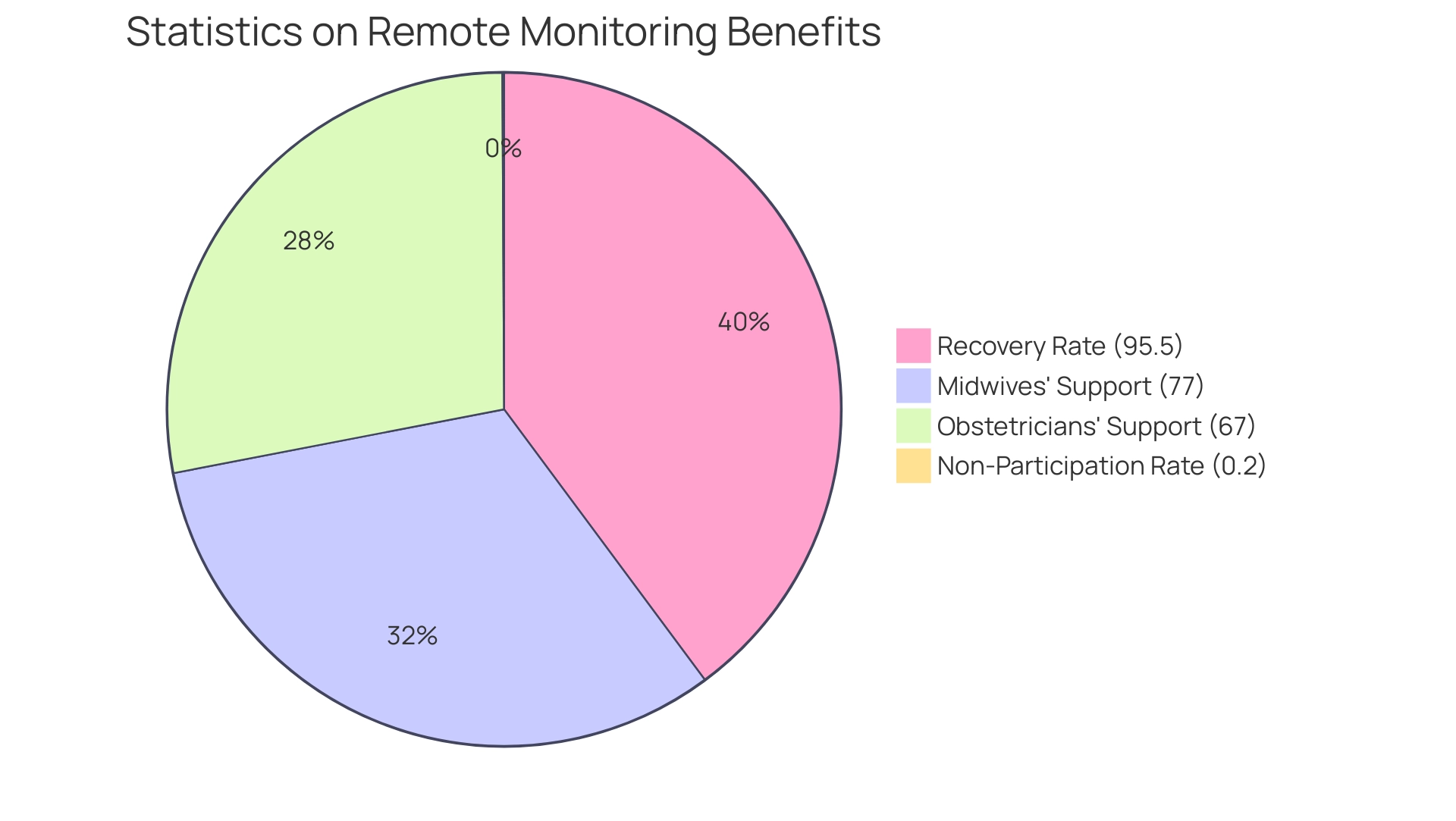
- 95.5% of individuals observed through distance health tracking for COVID-19 completely recovered.
- Only 0.2% did not participate for the necessary period.
Additionally, the practice of remote monitoring in clinical trials: benefits and challenges promotes consistent interaction between individuals and healthcare professionals, fostering a cooperative care atmosphere that improves overall health results. The significance of these technologies is underscored by the expanding global distance health tracking market, which relates to Remote Monitoring in Clinical Trials: Benefits and Challenges, and is expected to rise from $14 billion in 2023 to around $42 billion by 2028.
Additionally, a quote from Strategic Market Research highlights that admissions for chronic care complications were reduced between 19% and 41% thanks to Remote Monitoring in Clinical Trials: Benefits and Challenges, which emphasizes its effectiveness. The Centers for Medicare and Medicaid Services (CMS) reported a staggering 1,298% increase in claims related to Remote Monitoring in Clinical Trials: Benefits and Challenges from 2019 to 2022, highlighting the system's growing adoption and the demonstrated advantages in both cost savings and care. Moreover, 77% of midwives and 67% of obstetricians think that distance oversight aids their clients, especially those at high risk, highlighting professional support for these technologies.
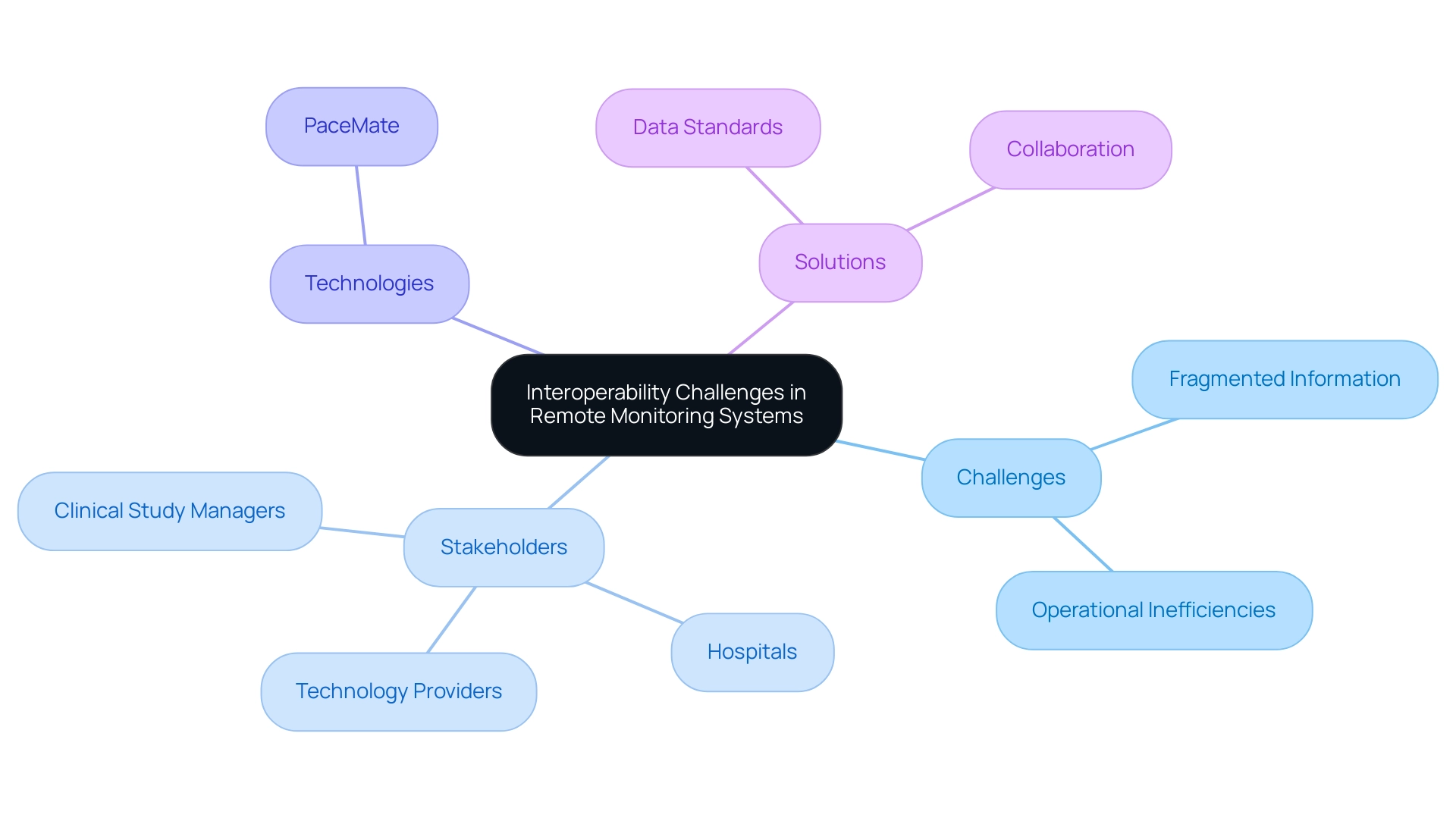
Navigating Interoperability Challenges in Remote Monitoring Systems
Interoperability issues in distant observation systems present considerable hurdles for clinical studies, often leading to fragmented information and operational inefficiencies, which highlights the Remote Monitoring in Clinical Trials: Benefits and Challenges. A diverse array of devices and software platforms frequently struggle to communicate effectively, which can lead to data silos that complicate patient observation and data analysis. Insights gathered from interviews with leaders in acute care and children’s hospitals, primary care providers, behavioral health providers, and regional health information exchange networks reveal a shared recognition of these challenges.
To successfully navigate this environment, clinical study managers must emphasize the adoption of interoperable technologies, such as PaceMate, which provides a scalable and efficient cardiac oversight solution that benefits from interoperability and integrates smoothly with existing systems. Creating strong data standards and protocols is essential for improving communication among diverse devices, thus maximizing the effectiveness of distant oversight initiatives. Additionally, collaboration with technology providers is essential to ensure compatibility and facilitate smoother integration.
As Runkle et al. observed, only about half of practitioners think these technologies will be more widely adopted in the future and could benefit their patients, emphasizing the essential requirement for continuous discussion and innovation to effectively tackle interoperability issues and realize the complete potential of remote monitoring in clinical trials: benefits and challenges.
Improving Oversight and Risk Management with Remote Monitoring
Remote supervision is transforming oversight and risk management in clinical studies by facilitating real-time data access and centralized oversight capabilities. This technology equips clinical research managers with the tools needed to swiftly identify potential issues, such as protocol deviations or adverse events, thereby facilitating timely interventions. The execution of risk-based oversight strategies, alongside comprehensive clinical study management services like feasibility assessments, study set-up, project management, and compliance reviews, permits the optimal allocation of resources based on identified risks, further enhancing oversight efforts.
Recent data demonstrates that digitally enabled enrollment has surged from 26% (SD 28%) to an impressive 70% (SD 26%), underscoring the growing reliance on such technologies. Additionally, a research project carried out in partnership with beagle Co., Ltd. demonstrated the efficacy of distance observation systems in improving clinical study integrity. 'YT, president of Beagle Co., Ltd., stated, 'The incorporation of distance observation is not merely a trend; it’s a crucial transformation that guarantees the utmost standards of individual safety and data integrity in clinical studies.'
Notably, the accuracy of uploaded photographs revealed no illegible images and only 0.1% were found to be incomplete. During R2BM, 2.3% of source documents that were missed were detected, with only 0.1% undetected. Additionally, misfiled photographs were identified, with 2.6% in incorrect folders for the correct participant and 3.2% in incorrect participants' folders.
These advancements not only improve transparency but also offer stakeholders with continuous updates concerning progress and patient safety. The RBQM framework, comprising eight functional elements like initial and ongoing risk evaluations, centralized oversight, and comprehensive reporting systems, further demonstrates how distant observation integrates into broader risk management strategies. Ultimately, the integration of remote monitoring in clinical trials: benefits and challenges is essential for maintaining compliance with regulatory standards set by authorities like INVIMA and ensuring the overall integrity of the research process.
Conclusion
The integration of remote monitoring into clinical trials represents a significant advancement in the field of clinical research, offering a multitude of benefits that enhance both efficiency and patient care. By reducing costs through the elimination of unnecessary travel and on-site staff, remote monitoring streamlines data collection processes, leading to substantial financial savings. Studies have demonstrated that these technologies can yield up to 30% in savings during specific trial phases, alongside improved patient outcomes such as reduced hospitalizations and enhanced quality of life.
Moreover, remote monitoring is transforming patient recruitment and engagement. By facilitating participation from the comfort of home, particularly for individuals in underserved areas, it broadens access to clinical trials. The use of wearable devices and mobile applications fosters continuous engagement, resulting in higher retention rates and a more diverse participant pool. These factors underscore the potential for remote monitoring to not only enhance inclusivity but also to promote better adherence to treatment protocols.
Despite the challenges of interoperability, the adoption of compatible technologies is crucial for maximizing the effectiveness of remote monitoring systems. By establishing robust data standards and fostering collaboration with technology providers, clinical trial managers can navigate these complexities, ensuring seamless integration and communication across platforms.
Ultimately, the shift towards remote monitoring in clinical trials enhances oversight and risk management, allowing for real-time data access and timely interventions. This not only safeguards patient safety but also upholds the integrity of clinical research. As the landscape of clinical trials continues to evolve, embracing remote monitoring will be essential for organizations aiming to improve outcomes and maintain a competitive edge in the industry.
Frequently Asked Questions
What are the main benefits of remote monitoring in clinical trials?
Remote monitoring in clinical trials offers significant cost savings by reducing travel expenses, minimizing the need for on-site staff, and streamlining data collection processes. It can lead to savings of up to 30% during certain trial stages.
How does remote monitoring impact patient outcomes?
Studies have shown that remote monitoring can result in 87% fewer hospitalizations and 77% fewer fatalities, along with a per-patient cost decrease of $11,472 compared to standard care. It also improves quality-adjusted life-years by 0.013.
In what ways does remote monitoring enhance participant recruitment?
Remote monitoring improves participant recruitment by expanding access to clinical studies for individuals in rural areas or those with mobility issues. Technologies like mobile applications and wearable devices allow participants to engage in studies from home, leading to increased enrollment rates.
What statistics support the effectiveness of remote monitoring for recruitment?
A recent survey indicated that 82% of individuals with cancer were willing to participate in studies using wearable technology, and 85% preferred studies held at nearby facilities, highlighting the importance of local engagement and technology in recruitment efforts.
How does remote monitoring affect participant retention in clinical trials?
Remote monitoring promotes ongoing participant involvement through regular check-ins and feedback, which enhances engagement and compliance with study protocols, resulting in increased retention rates.
What role does technology play in enhancing inclusivity in clinical research?
Innovative online strategies and remote monitoring help recruit diverse samples for randomized controlled studies, addressing logistical barriers that have historically limited participation among minorities and people of color.
How does remote monitoring contribute to cost efficiency in clinical trials?
By utilizing decentralized clinical study models, remote monitoring reduces reliance on physical locations and in-person visits, leading to an overall reduction in clinical study expenses by approximately 30%.




