Overview
This article delves into the pivotal insights and advancements regarding post-market studies in Paraguay, specifically underscoring the significance of bioaccess® as a preeminent contract research organization (CRO) within this domain. It asserts the critical nature of collaboration between CROs and Medtech companies, highlights the shifting regulatory landscape, and examines the integration of technology and real-world data. These elements collectively enhance the effectiveness and efficiency of post-market evaluations, ultimately leading to improved patient outcomes and optimized product lifecycle management.
Introduction
In the rapidly evolving landscape of medical technology, the significance of post-market studies is paramount. As companies strive to ensure the safety and efficacy of their devices, organizations like bioaccess® emerge as pivotal players, expertly navigating the complexities of clinical research in Paraguay.
With over 15 years of expertise, bioaccess® distinguishes itself as the leading contract research organization (CRO) specializing in post-market studies, offering a comprehensive suite of services tailored to meet the unique needs of Medtech companies.
As the demand for robust post-market clinical follow-up studies (PMCF) continues to escalate, a thorough understanding of the regulatory landscape, ethical considerations, and the integration of real-world data becomes essential for driving innovation and improving patient outcomes.
This article explores the significant role of bioaccess® in shaping the future of post-market studies in Paraguay, emphasizing the challenges and opportunities that lie ahead for the Medtech industry.
bioaccess®: Leading CRO for Post-Market Studies in Paraguay
bioaccess® is recognized as the premier contract research organization (CRO) in Paraguay, specializing in post-market studies in Paraguay for medical devices. With over 15 years of experience in the Medtech sector, bioaccess® provides a comprehensive array of services designed to streamline the journey of medical technologies from initial research to market entry. Their profound understanding of local regulatory frameworks, coupled with a commitment to delivering high-quality research, positions them as an essential partner for Medtech companies navigating the complexities of post-market follow-up assessments (PMCF), including the reporting of assessment status and adverse events.
In 2025, the landscape for post-market studies in Paraguay is transforming, featuring improved collaboration between sponsors and CROs to ensure the safety and efficacy of new medical devices. This trend underscores Paraguay's growing reputation as a reliable location for research trials, where companies can find an informed environment conducive to quality information gathering. As Julio Martinez-Clark remarked, "Colombia’s reputation has evolved into a knowledgeable country for companies to visit, feel safe/comfortable, and find patients to conduct trials for quality data," a sentiment that resonates within the broader regional context applicable to Paraguay, highlighting bioaccess's pivotal role in cultivating such an atmosphere.
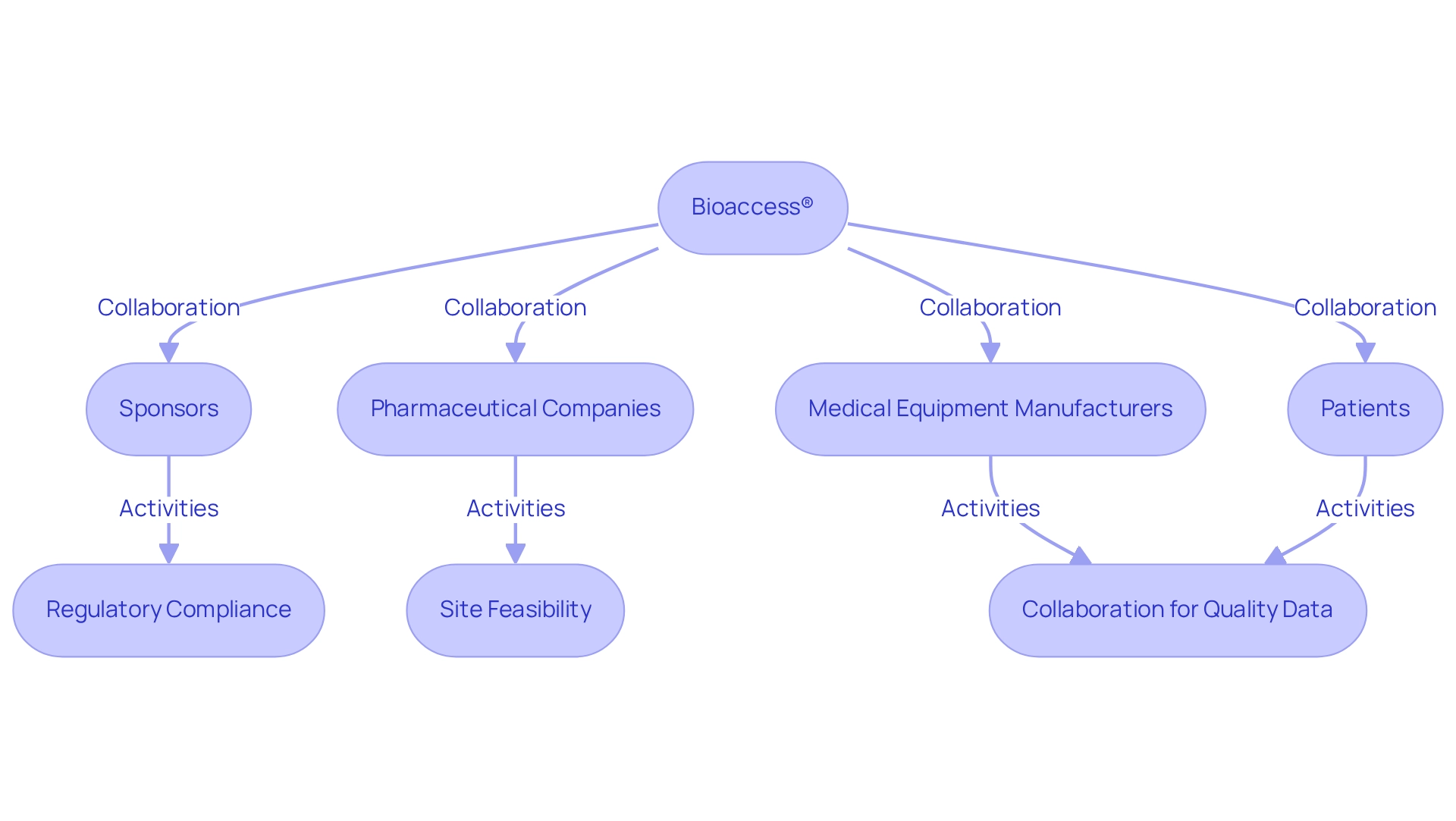
The market share of bioaccess® within Paraguay's CRO sector illustrates their significant contribution to advancing post-market studies in Paraguay, utilizing their expertise to enhance the overall quality of medical research. Case analyses reveal the successful implementation of post-market studies in Paraguay, showcasing bioaccess®'s effective collaboration with various stakeholders, including pharmaceutical companies and medical equipment manufacturers. For example, Avantec Vascular's first-in-human clinical trial of an innovative vascular device in Latin America was supported by bioaccess®, demonstrating their proficiency in site feasibility, investigator selection, and regulatory compliance. Such collaborations not only elevate the quality of data but also stimulate innovation within the Medtech industry. Insights from Novotech's case analyses further highlight the importance of these partnerships. As the demand for robust post-market studies in Paraguay continues to escalate, bioaccess® remains at the forefront, spearheading advancements that ultimately enhance patient outcomes and bolster the growth of medical technologies in Latin America.
Understanding Paraguay's Regulatory Landscape for Medical Devices
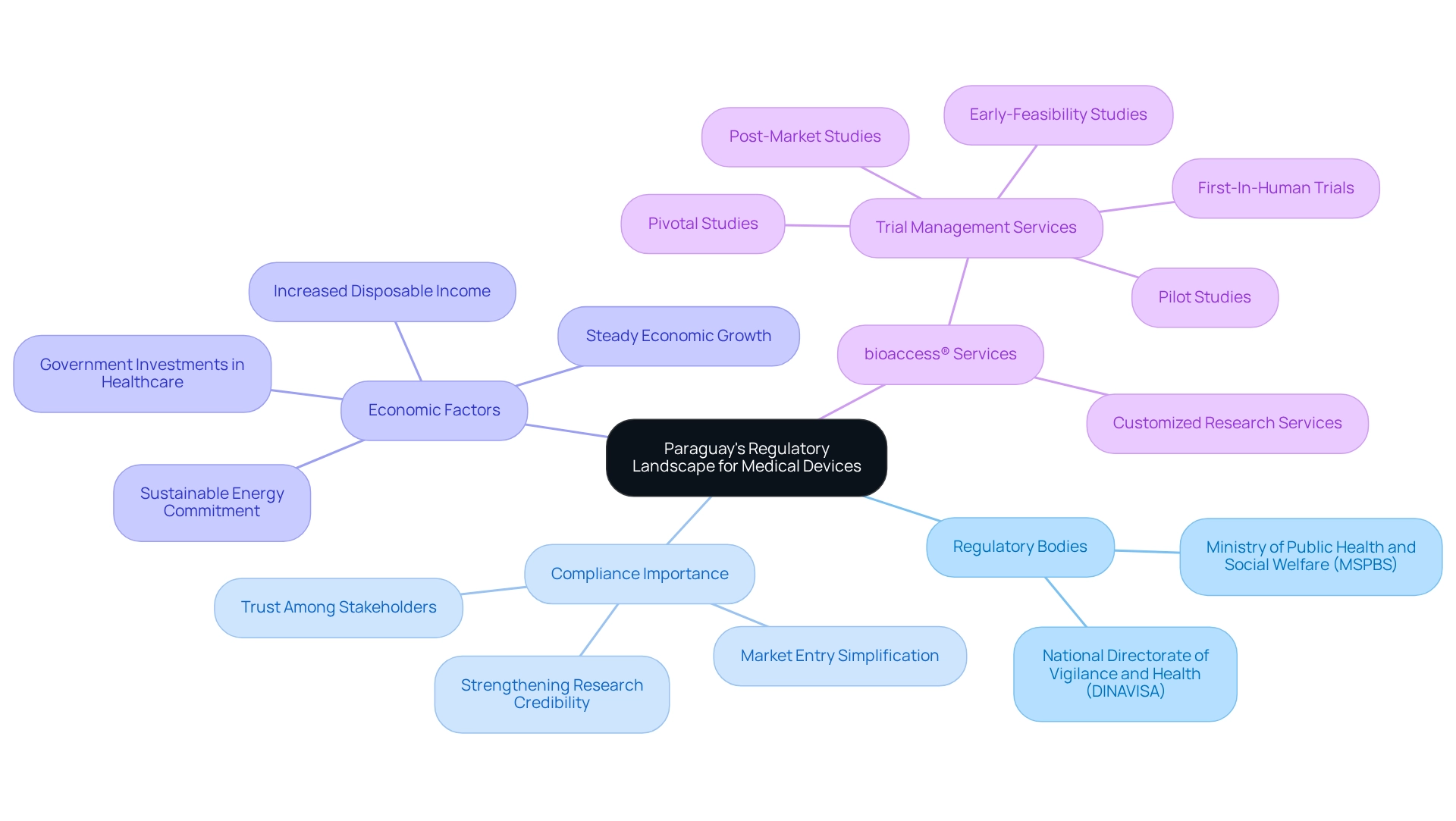
Paraguay's regulatory framework for medical equipment is primarily overseen by the Ministry of Public Health and Social Welfare (MSPBS) and the National Directorate of Vigilance and Health (DINAVISA). Recent regulatory updates have aligned local practices with international standards, ensuring that medical products adhere to stringent safety and efficacy requirements. For Medtech firms, a comprehensive grasp of these regulations is crucial, as they oversee the procedures for device registration, post-market studies in Paraguay, and follow-up evaluations. Complying with these guidelines not only simplifies market entry but also strengthens the credibility of research studies, ultimately promoting trust among stakeholders and improving the likelihood of successful product acceptance in the expanding Paraguayan market.
bioaccess® is dedicated to ensuring information security and client confidence through strong information protection measures. Our grievance and data protection procedures are intended to address client concerns with compliance and transparency, reinforcing our dedication to ethical practices in research. If you have any queries or concerns about the processing of your information, you may email our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), 1200 Brickell Avenue, Suite 1950 #1034, email: info@bioaccessla.com.
The Paraguayan economy has been growing steadily, leading to increased disposable income and improved access to healthcare services. Government investments in healthcare infrastructure are promoting the use of advanced medical technologies, creating a favorable environment for Medtech companies. Furthermore, WHO's recent recommendations emphasize the need for improved trial design, greater diversity of participants, and efficient infrastructure, which are critical for regulatory compliance.
Furthermore, Paraguay's dedication to sustainable energy generation fosters improvements in healthcare infrastructure, indirectly influencing the regulatory landscape for medical equipment. However, Medtech companies must also navigate various regulatory compliance challenges, which can impact their market strategies and operational effectiveness. With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-market studies in Paraguay, we provide comprehensive trial management services that effectively navigate these complexities. Our expedited medical equipment clinical research services are customized to address the distinct requirements of the Latin American market.
Significance of Post-Market Clinical Follow-Up Studies (PMCF)
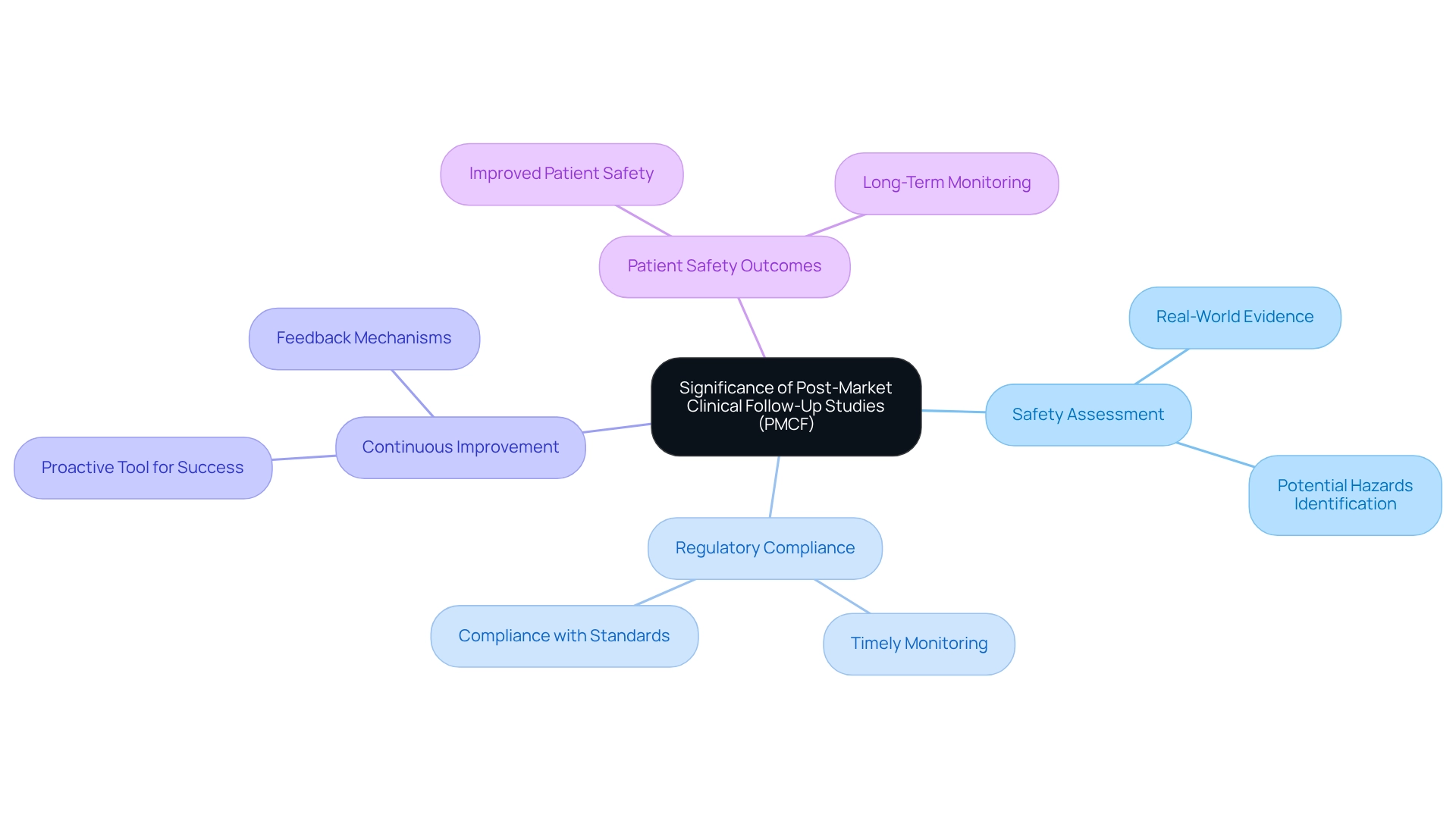
Post-Market Clinical Follow-Up Studies (PMCF) are essential for the ongoing assessment of medical devices' safety and performance after market approval. These investigations yield crucial information that can uncover potential hazards and guide necessary adjustments to product application or labeling. By systematically gathering real-world evidence, PMCF research assists manufacturers in demonstrating compliance with regulatory standards and significantly enhances patient safety. In 2025, the importance of these analyses is underscored by the fact that 20.1% of the 855,057 injury reports for products without ongoing recalls were submitted late, highlighting the urgent need for timely monitoring and responsive mechanisms.
Moreover, PMCF analyses contribute to the robust clinical evidence that substantiates a product's effectiveness, which is critical for maintaining market authorization. Expert opinions emphasize that when PMCF is embraced as a proactive tool for continuous improvement, it positions medical products for success in the evolving healthcare landscape, aligning with insights from NSF. Real-world examples illustrate how PMCF initiatives have led to improved patient safety outcomes, underscoring their significance in the medical device sector.
At bioaccess®, we specialize in managing PMCF projects alongside Early-Feasibility Projects (EFS), First-In-Human Trials (FIH), and Pilot Trials, leveraging over 20 years of Medtech experience to ensure these projects are executed efficiently and effectively. Our comprehensive clinical trial management services encompass feasibility assessments, site selection, compliance reviews, trial setup, import permits, project management, and reporting, all tailored to meet the unique demands of the Latin American market. Additionally, a well-structured PMS plan should outline data collection methods, analysis processes, actions for safety concerns, and revision timelines, providing a thorough overview of PMCF initiatives and their implementation. As the industry evolves, the role of PMCF evaluations in long-term safety monitoring becomes increasingly vital, ensuring that devices not only satisfy initial safety standards but continue to function effectively throughout their lifecycle.
Challenges in Conducting Post-Market Studies in Paraguay
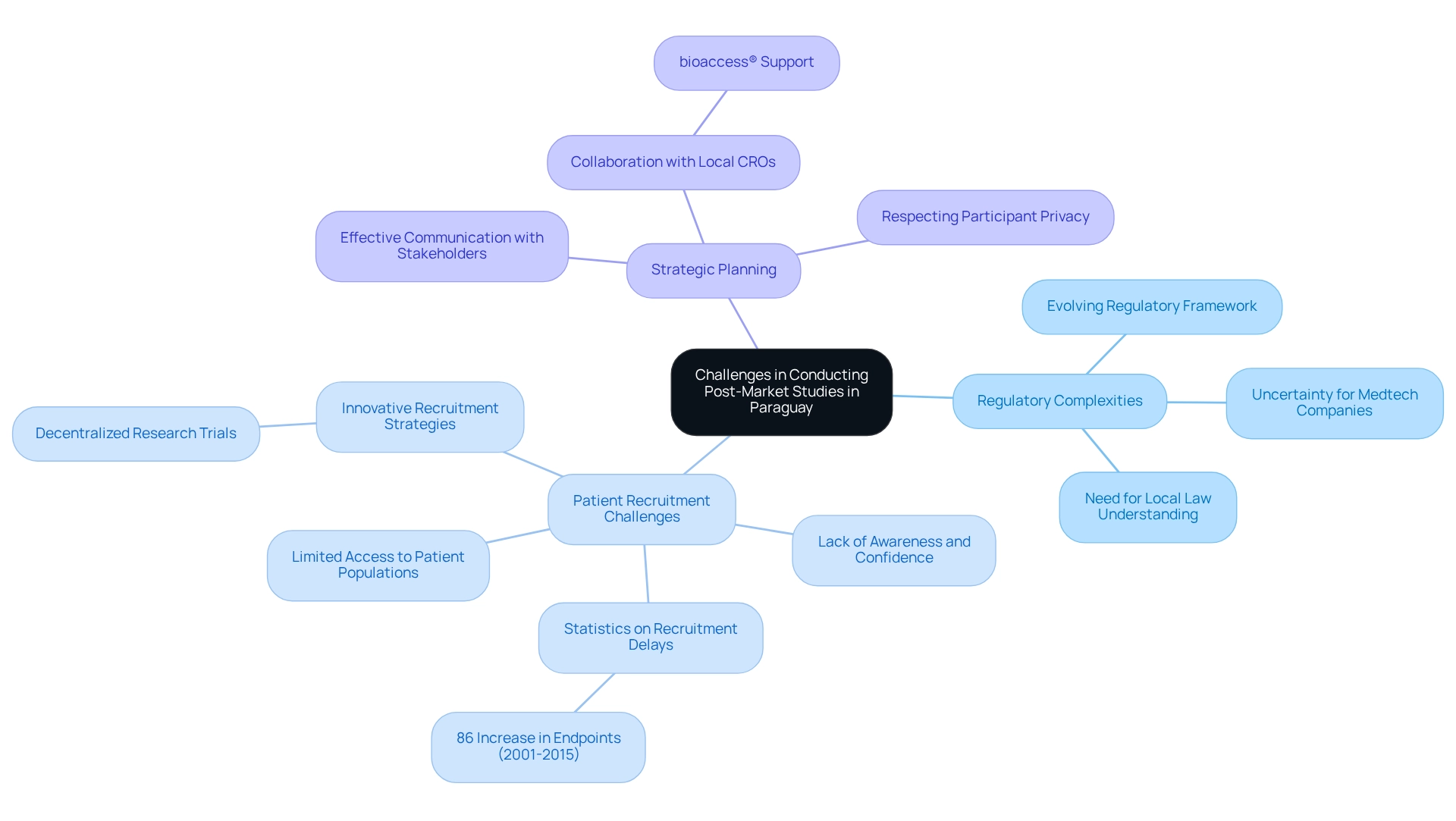
Carrying out post-market studies in Paraguay involves navigating a landscape fraught with significant challenges. At the forefront are regulatory complexities; the evolving framework can introduce uncertainty for Medtech companies striving to meet new requirements. This is especially relevant in 2025, as the regulatory environment continues to change, necessitating a thorough understanding of local laws and guidelines.
Compounding these difficulties is the limited access to patient populations, which complicates the recruitment process. Many potential participants may lack awareness or confidence in medical research, hindering enrollment efforts. Statistics indicate that patient recruitment challenges are a common issue in research trials, with numerous studies experiencing delays due to insufficient participant numbers. Notably, the total count of endpoints in trial protocols has surged by 86% from 2001 to 2015, underscoring the increasing complexity of research and the need for diverse participant groups. This complexity accentuates the importance of innovative recruitment strategies, such as those seen in decentralized research trials, which have gained prominence in recent years. These trials utilize local resources and technology to enhance participant engagement and streamline the recruitment process.
To surmount these challenges, strategic planning and effective communication with stakeholders are crucial. Collaborating with local contract research organizations (CROs) like bioaccess® can provide invaluable assistance in navigating the regulatory landscape and improving recruitment strategies. bioaccess® has demonstrated its capability in managing various trials, including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-market studies in Paraguay, significantly increasing the likelihood of successful execution. By leveraging local expertise and resources, Medtech companies can bolster their recruitment efforts, ultimately leading to improved outcomes for both the research and the patients involved. Furthermore, it is imperative to respect participants' privacy during recruitment, ensuring that ethical considerations remain a priority in medical research.
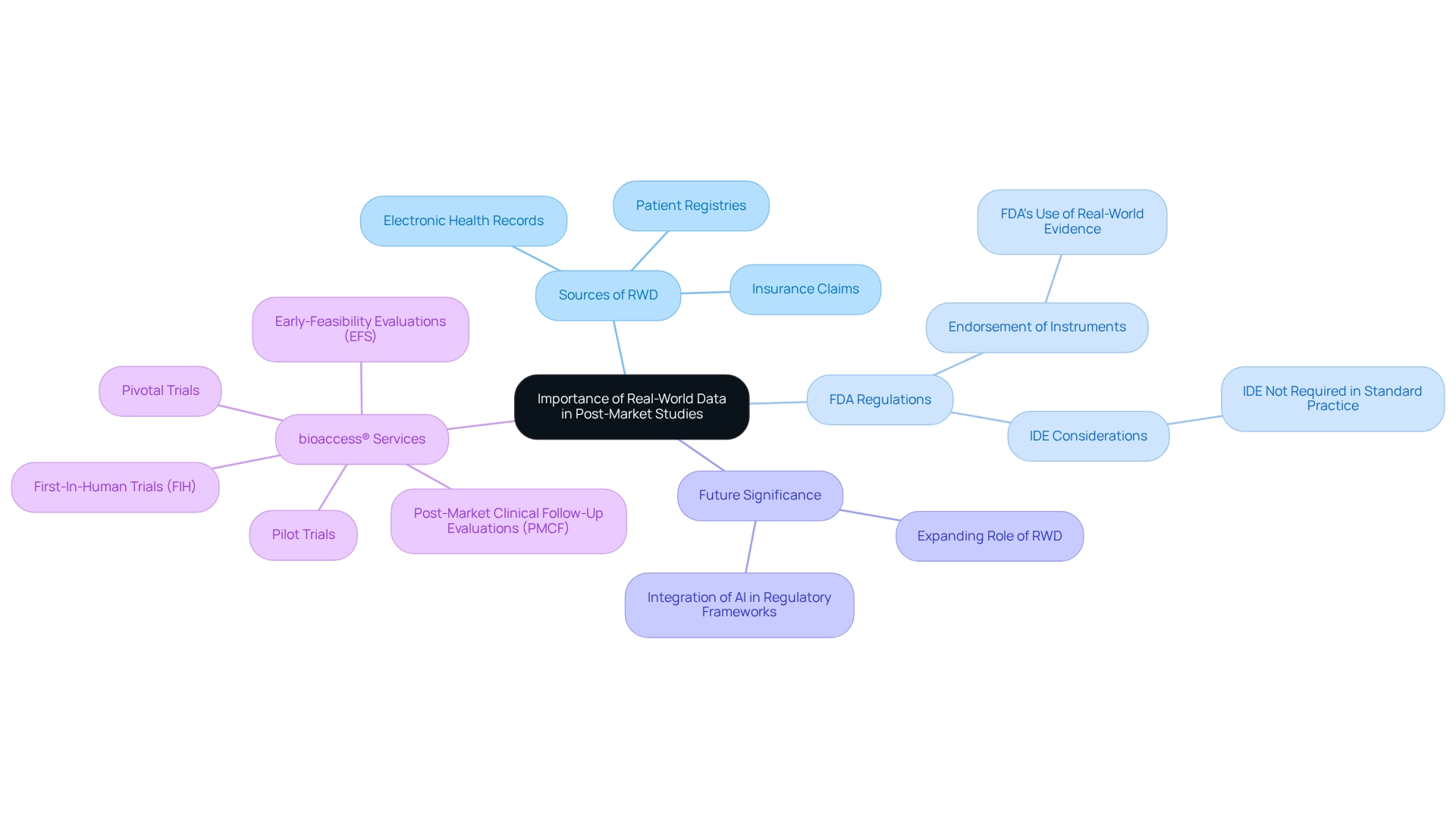
Importance of Real-World Data in Post-Market Studies
Real-world information (RWI) plays a pivotal role in post-market research, offering essential insights into the effectiveness of medical instruments in everyday clinical settings. Researchers can obtain real-world data (RWD) from sources such as:
- electronic health records
- patient registries
- insurance claims
This enables them to assess the long-term safety and effectiveness of medical equipment. This data is vital for identifying potential adverse events and understanding patient outcomes, ultimately enhancing the robustness of study findings. Notably, the FDA's endorsement of a medical instrument indication based solely on real-world evidence in June 2017 underscores the agency's increasing reliance on RWE, which can streamline regulatory procedures and reduce the need for new clinical trials.
Furthermore, the FDA has indicated that an Investigational Device Exemption (IDE) may not be necessary if an instrument is utilized within the standard scope of medical practice, emphasizing the importance of RWD in substantiating claims of safety and effectiveness while ensuring compliance with eIFU guidelines established by the EU and FDA. As the landscape of medical device research evolves, the integration of RWD into post-market studies in Paraguay enhances regulatory submissions and fosters a deeper understanding of device performance in real-world environments.
Looking ahead to 2025, the significance of RWD will continue to expand, particularly as regulatory frameworks adapt to incorporate innovative technologies, including AI-driven decision support systems for healthcare. By harnessing RWD, Medtech firms can ensure their research is comprehensive and aligned with current regulatory expectations, ultimately contributing to market success.
bioaccess® specializes in expedited medical apparatus research services in Latin America, focusing on:
- Early-Feasibility Evaluations (EFS)
- First-In-Human Trials (FIH)
- Pilot Trials
- Pivotal Trials
- Post-Market Clinical Follow-Up Evaluations (PMCF)
This expertise ensures that RWD is effectively utilized to enhance the credibility and efficacy of these analyses. As Chris Rush, a Solutions Engineer with extensive experience overseeing trials for Class III products, notes, the incorporation of RWD is essential for bolstering the credibility and effectiveness of post-market studies in Paraguay. To leverage RWD effectively, researchers should prioritize establishing robust data collection methods early in their investigations, ensuring comprehensive insights into device performance.
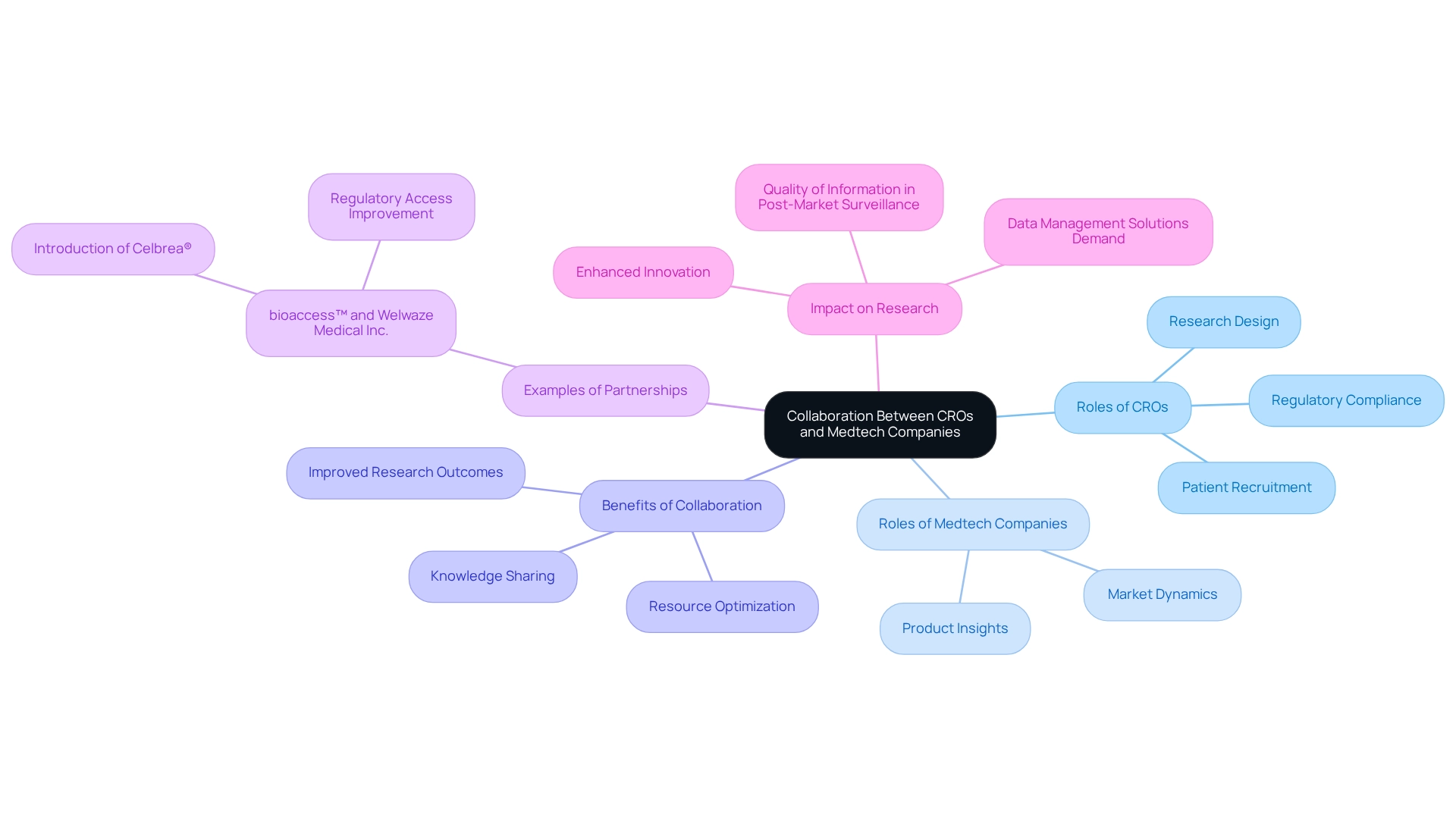
Collaboration Between CROs and Medtech Companies for Effective Studies
Collaboration between contract research organizations (CROs) like bioaccess™ and Medtech companies is crucial for the successful implementation of post-market assessments. Julio Martinez-Clark, CEO of bioaccess™, advocates for Medtech clinical research in Latin America, emphasizing the region's potential for operationalizing clinical trials.
With over a decade of experience supporting Medtech companies and his role as chairman of the board of the Association for the Advancement of Clinical Research in Colombia (AVANZAR), Julio brings significant expertise to the field. CROs provide specialized expertise in regulatory compliance, research design, and patient recruitment, while Medtech companies offer valuable insights into their products and market dynamics.
This synergistic partnership cultivates an environment conducive to knowledge sharing and resource optimization, ultimately leading to more effective studies. For instance, bioaccess™ has partnered with Welwaze Medical Inc. to support the introduction of the Celbrea® medical equipment in Colombia, demonstrating how strategic collaborations can improve regulatory access and market entry.
By collaborating, both parties can address challenges, optimize processes, and significantly improve the quality of information gathered during post-market surveillance. Effective partnerships not only enhance research outcomes but also promote innovation in the Medtech sector, ensuring that new devices satisfy market demands and regulatory standards efficiently.
Moreover, as the pharmaceutical, biotechnology, and healthcare industries continue to expand, the need for effective data management solutions in post-market evaluations rises. Notably, companies promoting teamwork experience a 50% lower employee turnover rate, highlighting the broader benefits of collaboration in clinical research settings.
To navigate the complexities of post-market research and achieve success, fostering a collaborative spirit is essential.
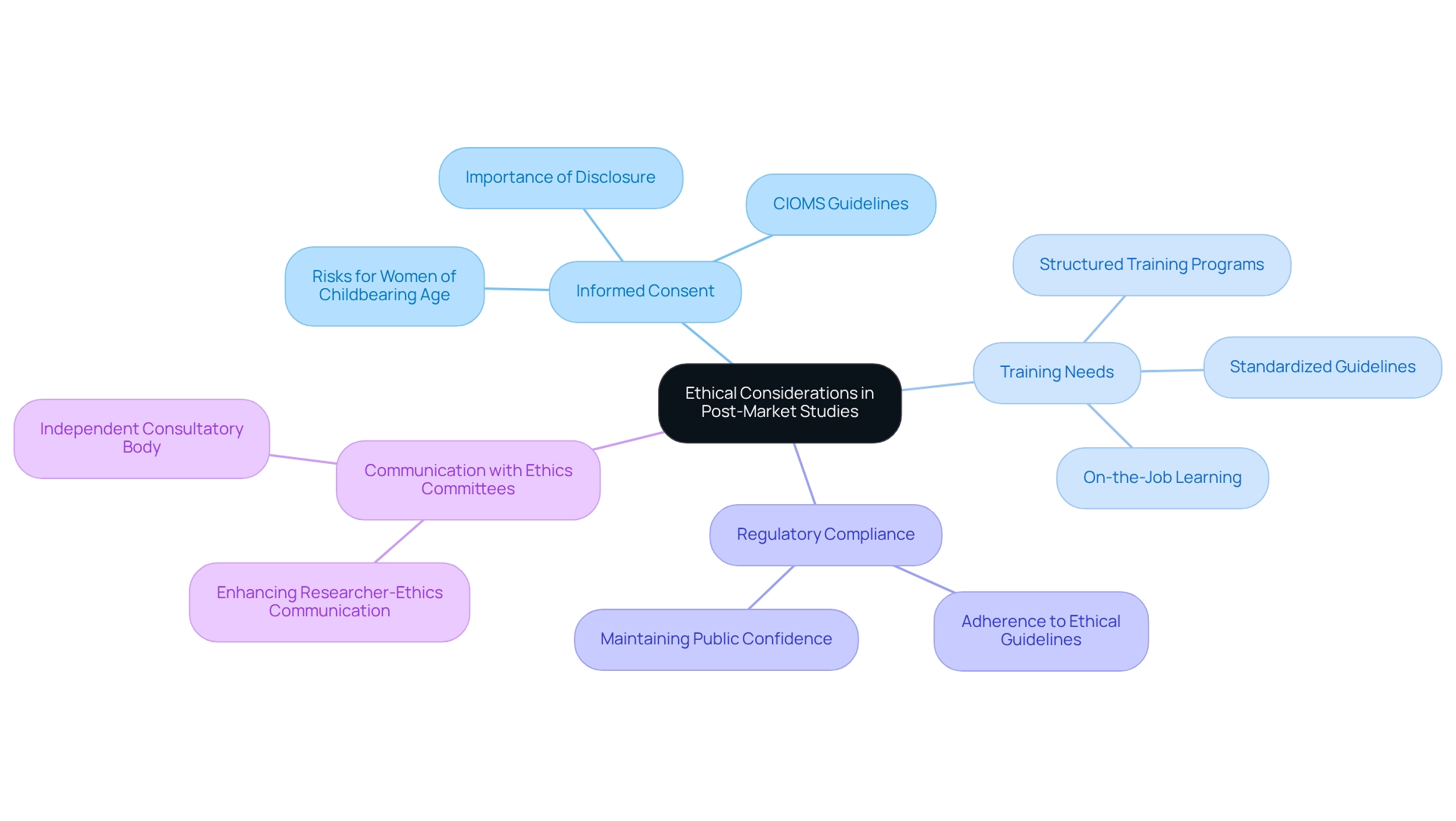
Ethical Considerations in Post-Market Studies
Ethical considerations are paramount in post-market studies in Paraguay, as these studies entail continuous oversight of patient safety and product effectiveness. Researchers must prioritize obtaining informed consent from participants, safeguarding their rights and well-being throughout the research process. Notably, a substantial number of researchers indicate that their understanding of informed consent is primarily derived from on-the-job experience. This highlights the urgent need for structured training and comprehensive guidelines to standardize practices across research groups. Furthermore, providing adequate time and opportunities for dialogue is essential for participants to make informed decisions regarding trial involvement.
Clarity in disclosing adverse events and research findings is critical for maintaining public confidence and meeting regulatory obligations. Adhering to established ethical guidelines not only protects participants but also enhances the credibility of the research, ultimately advancing medical knowledge and improving patient care. For example, the CIOMS guidelines specifically address informed consent for women of childbearing age, ensuring they are fully informed about potential risks related to pregnancy and reproductive health. This approach fosters informed decision-making and reinforces the ethical framework necessary for successful post-market studies in Paraguay.
As Antonia Xu suggests, the establishment of an independent consultatory body to address issues surrounding informed consent could significantly enhance communication between researchers and ethics review committees in the context of post-market studies in Paraguay. This, in turn, would support researchers in developing effective informed consent procedures. By implementing these best practices, clinical researchers can strengthen ethical considerations in post-market studies in Paraguay, fostering greater trust and engagement from participants.
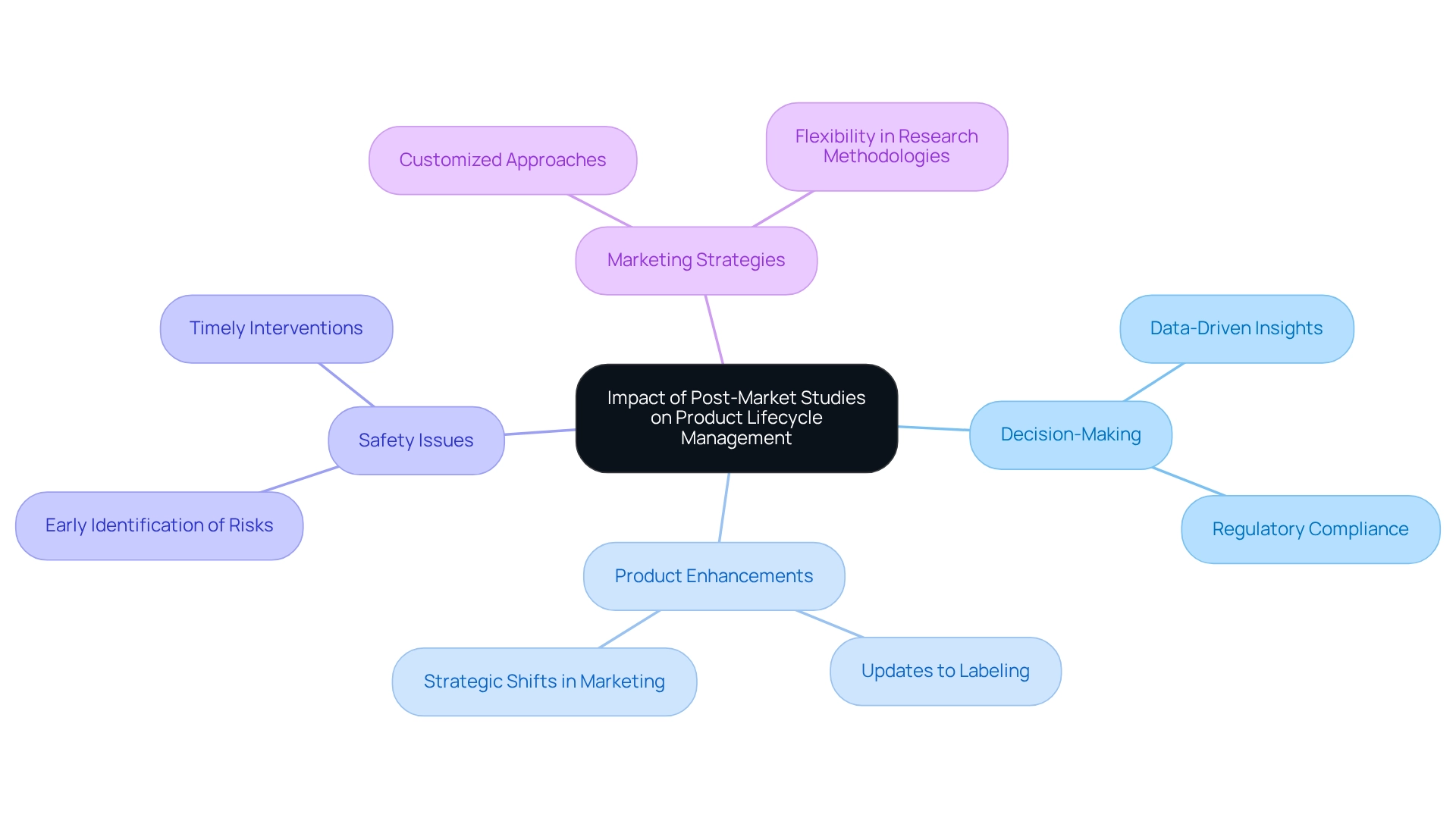
Impact of Post-Market Studies on Product Lifecycle Management
Post-market studies in Paraguay are essential in product lifecycle management, providing crucial information that guides decision-making throughout the lifespan of a medical device. Insights gained from these analyses can lead to significant product enhancements, updates to labeling, and strategic shifts in marketing approaches. Moreover, post-market studies in Paraguay enable manufacturers to identify potential safety issues early, allowing for timely interventions that can reduce risks. For instance, a recent analysis highlighted the flexibility in post-marketing requirements, allowing manufacturers to employ various methods for gathering safety information, showcasing the FDA's acknowledgment of diverse research designs while prioritizing drug safety. This flexibility is vital, as it allows manufacturers to customize their strategies to meet specific product demands and regulatory requirements.
In 2025, the impact of post-market studies in Paraguay on product lifecycle management is more pronounced than ever, with statistics indicating that companies leveraging data-driven insights are better positioned for success. Organizations with data-literate personnel are more likely to excel in this data-centric landscape, emphasizing the importance of incorporating post-market studies in Paraguay into lifecycle strategies. As Jon Speer, a medical equipment expert, articulates, "That's why he created Greenlight Guru to help companies move beyond compliance to True Quality." By embracing this approach, Medtech companies can not only improve product safety and optimize performance but also ensure adherence to evolving regulatory standards, ultimately enhancing patient outcomes and sustaining market success. Additionally, bioaccess® offers expedited medical device clinical research services in Latin America, specializing in Early-Feasibility Assessments (EFS), First-In-Human Trials (FIH), Pilot Trials, Pivotal Trials, and Post-market studies in Paraguay, thereby further assisting manufacturers in navigating the complexities of product lifecycle management. The median impact factor of 12.8 for publication journals underscores the importance of high-quality research in informing these investigations, further emphasizing the necessity for comprehensive information gathering and analysis.
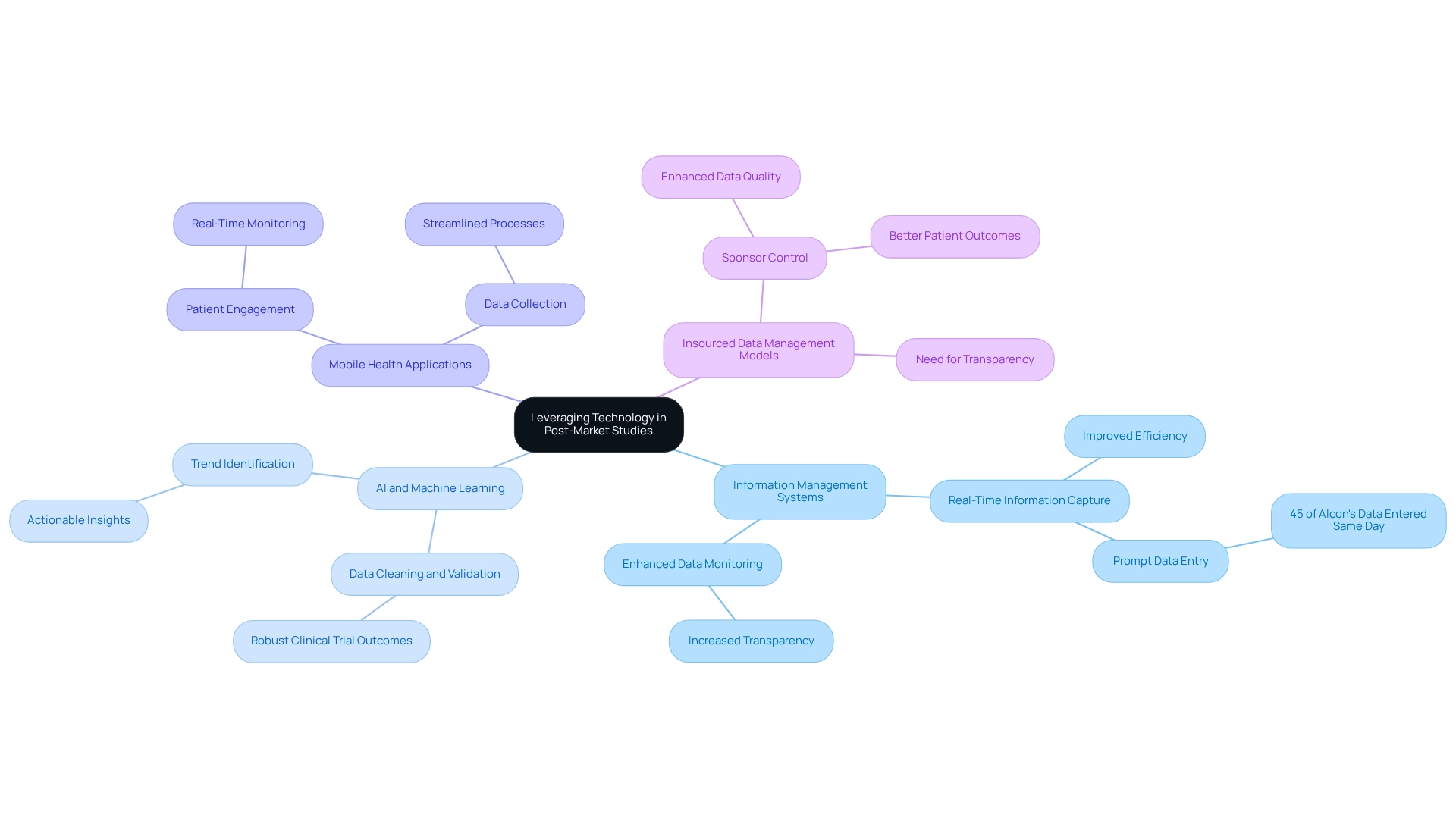
Leveraging Technology to Streamline Post-Market Studies
The incorporation of technology is essential for enhancing post-market evaluations and refining information gathering procedures within clinical research. Advanced information management systems, electronic health records, and mobile health applications enable real-time information capture and monitoring, significantly improving the efficiency of clinical research. For instance, 45% of Alcon's information is recorded on the same day as the visit date, underscoring the impact of prompt information entry on research efficiency.
Furthermore, the application of artificial intelligence and machine learning algorithms enhances analysis, allowing researchers to quickly identify trends and derive actionable insights. As noted by Michelle Crouthamel, Director of Digital Health and Innovation at AbbVie Inc., 'The balance of safeguarding research integrity and offering information feedback at suitable times is a crucial factor in research design.'
By embracing these technological advancements, Medtech companies like bioaccess®, with over 20 years of experience in the field, can enhance the quality of their post-market studies in Paraguay, such as:
- Early-Feasibility Studies
- First-In-Human Studies
- Post-Market Clinical Follow-Up Studies
This approach not only reduces operational costs but also expedites necessary product adjustments, ultimately facilitating a quicker time to market. Moreover, there is a growing trend for sponsors to take charge of their information management processes rather than relying solely on CROs, driven by the need for transparency and ownership. This shift towards insourced information management models improves the quality of information management and better delivers results for patients. The precision and speed of AI in cleaning and validating information also bolster the robustness of clinical trial outcomes, making it imperative for Medtech companies to leverage these technologies.
Future Trends in Post-Market Studies in Paraguay
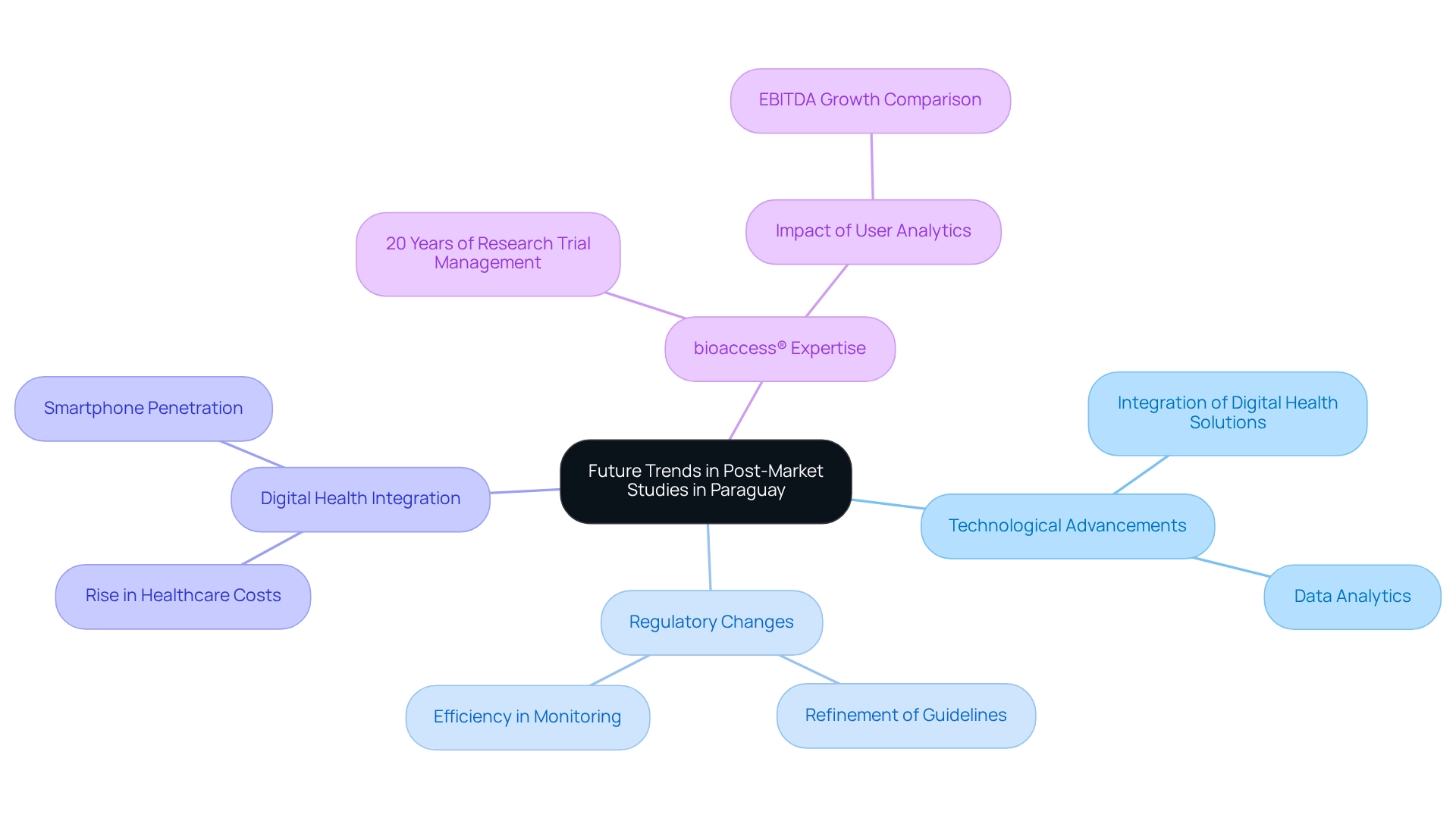
The future of post-market studies in Paraguay is positioned for transformative change, propelled by technological advancements and evolving regulatory frameworks. With the market anticipated to expand at a compound annual growth rate (CAGR) of 19.7% from 2025 to 2032, the demand for real-world evidence is on the rise. This shift necessitates a heightened focus on integrating digital health solutions and data analytics into post-market research methodologies. As healthcare delivery increasingly incorporates digital tools, Medtech companies, including bioaccess®, must harness these innovations to enhance their post-market surveillance processes.
bioaccess® brings over 20 years of expertise in managing research trials, including Early-Feasibility Trials (EFT), First-In-Human Trials (FIHT), Pilot Trials, and Post-Market Follow-Up Trials (PMFT). This extensive experience ensures that companies can navigate the complexities of regulatory compliance while achieving market success. Regulatory bodies are expected to further refine their guidelines, promoting efficiency in monitoring and evaluation. Organizations that proactively adapt to these trends will not only enhance their compliance but also gain a competitive edge in the research landscape. For instance, top-quartile users of analytics have demonstrated EBITDA growth 2.3 times greater than their bottom-quartile counterparts over five years, underscoring the financial advantages of adopting data-driven strategies, particularly in post-market evaluations.
Moreover, the integration of digital health technologies is becoming crucial for survival and growth in the data-driven healthcare environment. As Jignesh Rawal, Assistant Manager - Healthcare, remarked regarding Teladoc's new app, "the integration of services for primary care and mental health is a significant step towards comprehensive digital health solutions." This approach aligns with bioaccess's commitment to delivering innovative solutions in clinical research. Recent case analyses indicate that macroeconomic factors, such as rising healthcare costs and increased smartphone usage, are driving the acceptance of affordable digital health solutions. Traditional healthcare delivery methods, characterized by in-person consultations and paper-based records, are being supplanted by these innovative approaches. Medtech firms that capitalize on these trends, particularly those collaborating with bioaccess®, will be well-positioned to excel in post-market studies in Paraguay's evolving landscape.
Conclusion
The landscape of post-market studies in Paraguay is experiencing a significant transformation, propelled by the expertise of organizations like bioaccess® and the evolving regulatory environment. With over 15 years of experience, bioaccess® emerges as a leading contract research organization, proficient in navigating the complexities of post-market clinical follow-up studies (PMCF) and ensuring the safety and efficacy of medical devices. The increasing demand for robust PMCF studies underscores the necessity of collaboration between Medtech companies and CROs, fostering innovation and enhancing patient outcomes.
Understanding Paraguay's regulatory framework is essential for Medtech companies, as adherence to these guidelines not only streamlines market entry but also bolsters the credibility of clinical studies. The integration of real-world data (RWD) and technological advancements is revolutionizing the approach to post-market studies, facilitating more efficient data collection and analysis, ultimately leading to improved patient safety and device performance.
As challenges such as patient recruitment and regulatory complexities continue, strategic partnerships and innovative methodologies become vital for success. The future of post-market studies in Paraguay is promising, with anticipated growth fueled by data-driven strategies and digital health solutions. Companies that proactively embrace these trends will enhance compliance and secure a competitive advantage in the evolving Medtech landscape.
In conclusion, the role of bioaccess® in advancing post-market studies is pivotal, ensuring that Medtech companies can adeptly navigate the intricacies of the regulatory environment while prioritizing patient safety and product lifecycle management. By leveraging expertise, technology, and real-world insights, the Medtech industry in Paraguay is well-positioned to thrive, ultimately benefiting healthcare providers and patients alike.
Frequently Asked Questions
What is bioaccess® and what services do they provide?
bioaccess® is a premier contract research organization (CRO) in Paraguay, specializing in post-market studies for medical devices. They offer a comprehensive range of services to facilitate the journey of medical technologies from initial research to market entry, including post-market follow-up assessments (PMCF) and the reporting of assessment status and adverse events.
How does bioaccess® contribute to post-market studies in Paraguay?
With over 15 years of experience in the Medtech sector, bioaccess® enhances the quality of medical research in Paraguay by collaborating with various stakeholders, including pharmaceutical companies and medical equipment manufacturers. Their expertise helps in site feasibility, investigator selection, and regulatory compliance, thereby improving data quality and fostering innovation in the Medtech industry.
What is the regulatory framework for medical equipment in Paraguay?
The regulatory framework for medical equipment in Paraguay is overseen by the Ministry of Public Health and Social Welfare (MSPBS) and the National Directorate of Vigilance and Health (DINAVISA). Recent updates have aligned local practices with international standards, ensuring that medical products meet stringent safety and efficacy requirements.
Why are Post-Market Clinical Follow-Up Studies (PMCF) important?
PMCF studies are essential for the ongoing assessment of medical devices' safety and performance after market approval. They provide crucial information to identify potential hazards and guide necessary adjustments, thereby enhancing patient safety and ensuring compliance with regulatory standards.
What challenges do Medtech companies face in Paraguay?
Medtech companies in Paraguay must navigate various regulatory compliance challenges that can impact their market strategies and operational effectiveness. Understanding the local regulatory environment is crucial for successful device registration and post-market studies.
How does bioaccess® ensure information security and client confidence?
bioaccess® is committed to ensuring information security through strong protection measures and transparent grievance and data protection procedures. Clients can address their concerns by contacting the Grievance Officer via email or at their office address.
What role does bioaccess® play in the evolving landscape of post-market studies in Paraguay?
bioaccess® is at the forefront of advancements in post-market studies in Paraguay, facilitating improved collaboration between sponsors and CROs. Their efforts contribute to the growing reputation of Paraguay as a reliable location for research trials, ultimately enhancing patient outcomes and supporting the growth of medical technologies in Latin America.




