Overview
This article examines strategies for fostering Medtech innovation through research conducted in Peru. It underscores the critical role of collaboration with local stakeholders, the necessity of understanding regulatory frameworks, and the importance of integrating cultural insights. These elements are essential for enhancing the effectiveness of clinical trials and driving innovation within the medical technology sector.
Introduction
In the dynamic landscape of Peru's healthcare sector, a transformative wave is reshaping the Medtech industry through innovative clinical research. At the forefront of this movement is bioaccess®, a leader in delivering tailored clinical research services that expedite the development of medical devices. With a rich history spanning over 15 years, bioaccess® empowers Medtech startups by navigating the complexities of clinical trials, ensuring they overcome regulatory hurdles and recruitment challenges.
As the demand for advanced healthcare solutions surges, understanding the intricacies of Peru's regulatory landscape and engaging local stakeholders becomes paramount. This article delves into the crucial elements driving Medtech innovation in Peru, highlighting the significance of:
- Collaboration
- Cultural insights
- Strategic patient recruitment
in fostering a thriving clinical trial ecosystem.
bioaccess: Accelerating Medtech Innovation Through Clinical Research in Peru
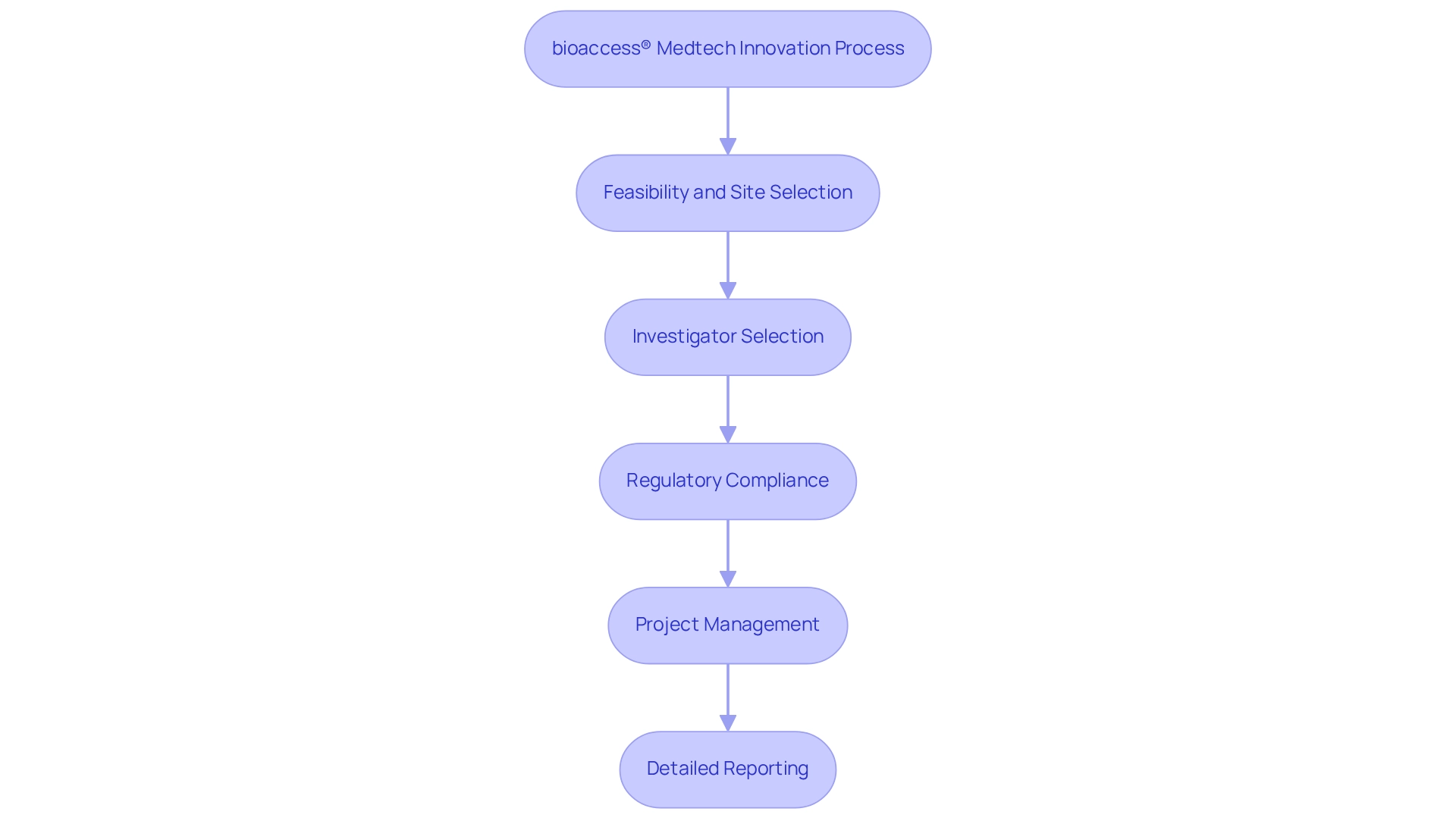
bioaccess® is at the forefront of Medtech innovation through Peruvian research, offering essential services that greatly accelerate the development of medical devices. With over 15 years of dedicated experience, bioaccess® excels in various research types, including first-in-human (FIH) and pivotal investigations, empowering medical technology startups to adeptly navigate the complexities of research processes. This tailored approach encompasses a comprehensive methodology that includes:
- Feasibility and selection of research sites
- Investigator selection
- Regulatory compliance
- Project management
- Detailed reporting
This ensures that startups can effectively tackle common challenges such as regulatory hurdles and recruitment issues.
The landscape of clinical trials in Peru has recorded a total of 26 entries from 1999 to 2024, which, although modest, indicates a growing commitment to advancing healthcare through research and highlights the potential for growth in the medical technology sector. Medical research organizations in the country play a crucial role in enhancing patient outcomes and elevating healthcare study standards. By leveraging local expertise and remaining attuned to the latest market intelligence, bioaccess® provides a strategic advantage for Medtech companies, enabling them to anticipate industry shifts and capitalize on emerging opportunities.
As emphasized in the Horizon Databook, the medical device trial market is replete with investment opportunities, underscoring the importance of informed decision-making in this sector. The impact of bioaccess® on medical device development in Peru highlights Medtech innovation through Peruvian research, as it not only fosters innovation but also contributes to economic growth and job creation. As noted by the bioaccess® content team, "Medical research can create jobs, promote economic growth, and enhance healthcare outcomes," highlighting the significance of international collaboration. Successful medical technology startups, such as Avantec Vascular, which partnered with bioaccess® for their initial human trial, have benefited from bioaccess®'s research services, showcasing the tangible advantages of collaboration in this evolving sector.
Understanding Peru's Regulatory Landscape for Medtech Trials
Comprehending Peru's Regulatory Framework for Medical Technology Studies
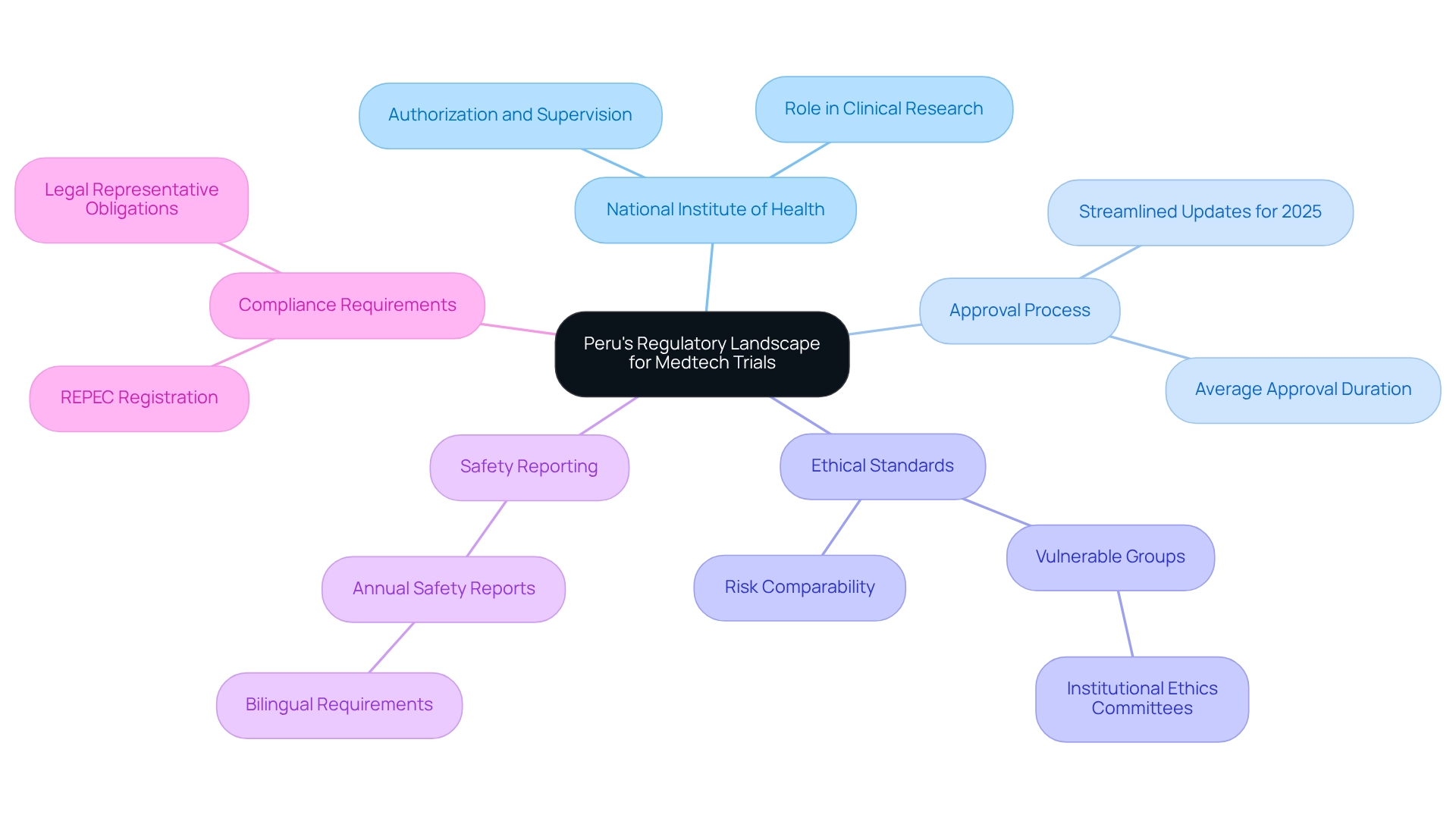
Peru's regulatory framework for medical technology studies is primarily overseen by the National Institute of Health (INS), which is responsible for the authorization and supervision of clinical research. A thorough understanding of the requirements for ethical approvals, safety reporting, and documentation is essential for medical technology firms aiming to navigate this landscape effectively.
Recent regulatory updates for 2025 have been implemented to streamline the approval process, making it increasingly accessible for international companies to conduct research in Peru. Notably, the average clinical study approval duration in Peru has significantly reduced, demonstrating the government's commitment to promoting innovation in the Medtech industry.
Furthermore, the INS mandates that sponsors provide yearly safety reports in both English and Spanish, ensuring clear communication and adherence. Additionally, it is crucial for the in-country legal representative to be registered in REPEC for the duration of the trial, which is a key aspect of regulatory compliance.
Ethical approvals are particularly stringent, especially for research involving vulnerable groups, such as prisoners, where institutional ethics committees must ensure that risks are comparable to those accepted by non-prisoner volunteers. This emphasis on ethical standards not only safeguards participants but also enhances the credibility of the research.
By educating themselves about these regulations, technology firms can ensure compliance and increase their likelihood of achieving successful study outcomes. The evolving regulatory framework in Peru presents a unique opportunity for Medtech innovation through Peruvian research, enabling innovative solutions to enter the market more swiftly and ultimately benefiting both the industry and patient care.
Utilizing the expertise of organizations such as bioaccess®, which focuses on extensive research management services—including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies—can further enhance the efficiency and effectiveness of navigating these regulatory challenges.
Engaging Local Stakeholders for Effective Clinical Trials in Peru
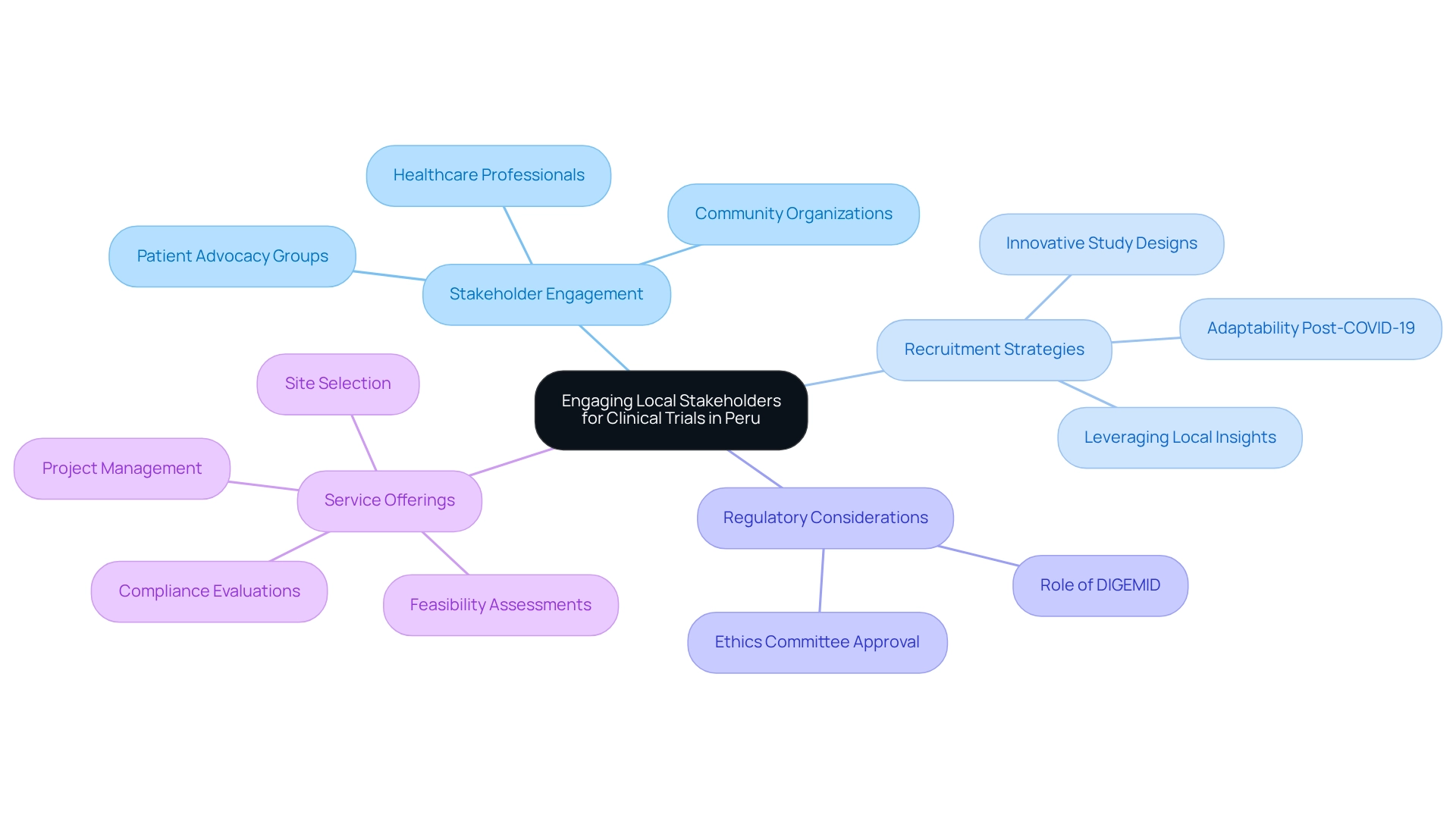
Successful medical studies in Peru hinge on the proactive engagement of local stakeholders, including healthcare professionals, community organizations, and patient advocacy groups. Collaborating with local hospitals not only boosts patient recruitment but also enhances retention rates. Involving these stakeholders from the onset of the design process ensures that studies are culturally relevant and tailored to community needs, which is vital for achieving positive outcomes. Notably, GlobalCare Clinical Trials' recent partnership with bioaccess™ to enhance ambulatory services in Colombia has resulted in over a 50% reduction in recruitment duration and a retention rate exceeding 95%. This achievement underscores the potential for Medtech innovation through Peruvian research, as a total of 26 medical studies have been conducted from 1999 to 2024, highlighting the critical need for strategic collaborations to increase this number.
Moreover, expert opinions emphasize that effective patient recruitment strategies, particularly those leveraging local insights, can significantly enhance enrollment success rates. The COVID-19 pandemic initially caused a slowdown in research participant enrollment; however, it also accelerated an industry-wide shift toward innovative study designs and creative patient recruitment strategies, demonstrating the necessary adaptability in this field. By cultivating robust relationships with local entities, Medtech companies can navigate the regulatory landscape more efficiently.
The General Directorate of Medicines, Supplies, and Drugs (DIGEMID) plays a pivotal role in the approval process for research studies, with their assessments being essential for ensuring that investigational products are both safe and effective. This collaborative approach not only adheres to regulatory standards, including the requirement for recruitment materials to be sanctioned by an ethics committee or institutional review board, but also enhances the overall quality and impact of research in the region.
Furthermore, bioaccess™ offers comprehensive services such as feasibility assessments, site selection, compliance evaluations, research preparation, import permits, project management, and reporting, which are crucial for the success of research initiatives.
Incorporating Cultural Insights into Medtech Clinical Trials
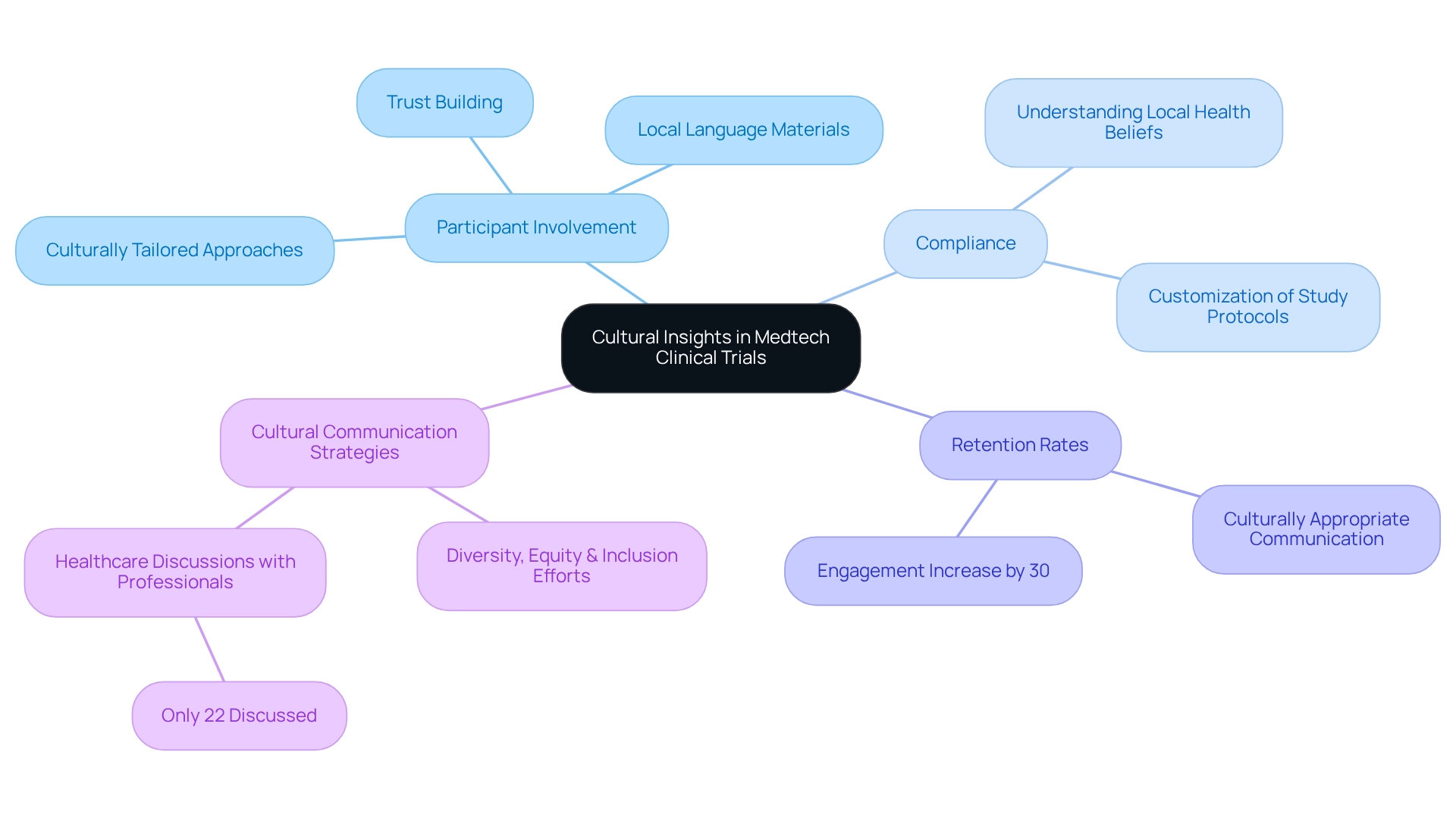
Integrating cultural insights into Medtech innovation through Peruvian research trials is essential for promoting participant involvement and ensuring compliance. A deep understanding of local health beliefs, practices, and preferences allows for the customization of study protocols that resonate with the target population. For instance, providing consent forms and educational materials in local languages not only enhances comprehension but also builds trust among participants. Furthermore, acknowledging traditional health practices can lead to more effective interactions, ultimately resulting in improved retention rates. Studies indicate that culturally tailored approaches can significantly boost participant retention, with data showing that such strategies can increase engagement by up to 30%.
Christine Chang, a research manager at Deloitte Services LP, emphasizes that integrating diversity, equity, and inclusion efforts is integral to advancing health equity in the biopharmaceutical and MedTech industries. This viewpoint underscores the importance of culturally appropriate communication strategies, particularly noting that only 22% of the group had research related to healthcare discussed with them by a professional. By prioritizing these cultural insights, Medtech companies like bioaccess®, which has over 20 years of experience in managing comprehensive research services—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—can achieve Medtech innovation through Peruvian research and enhance the effectiveness of their assessments. This not only contributes to more equitable health outcomes in the region but also supports local economies through job creation and healthcare improvement.
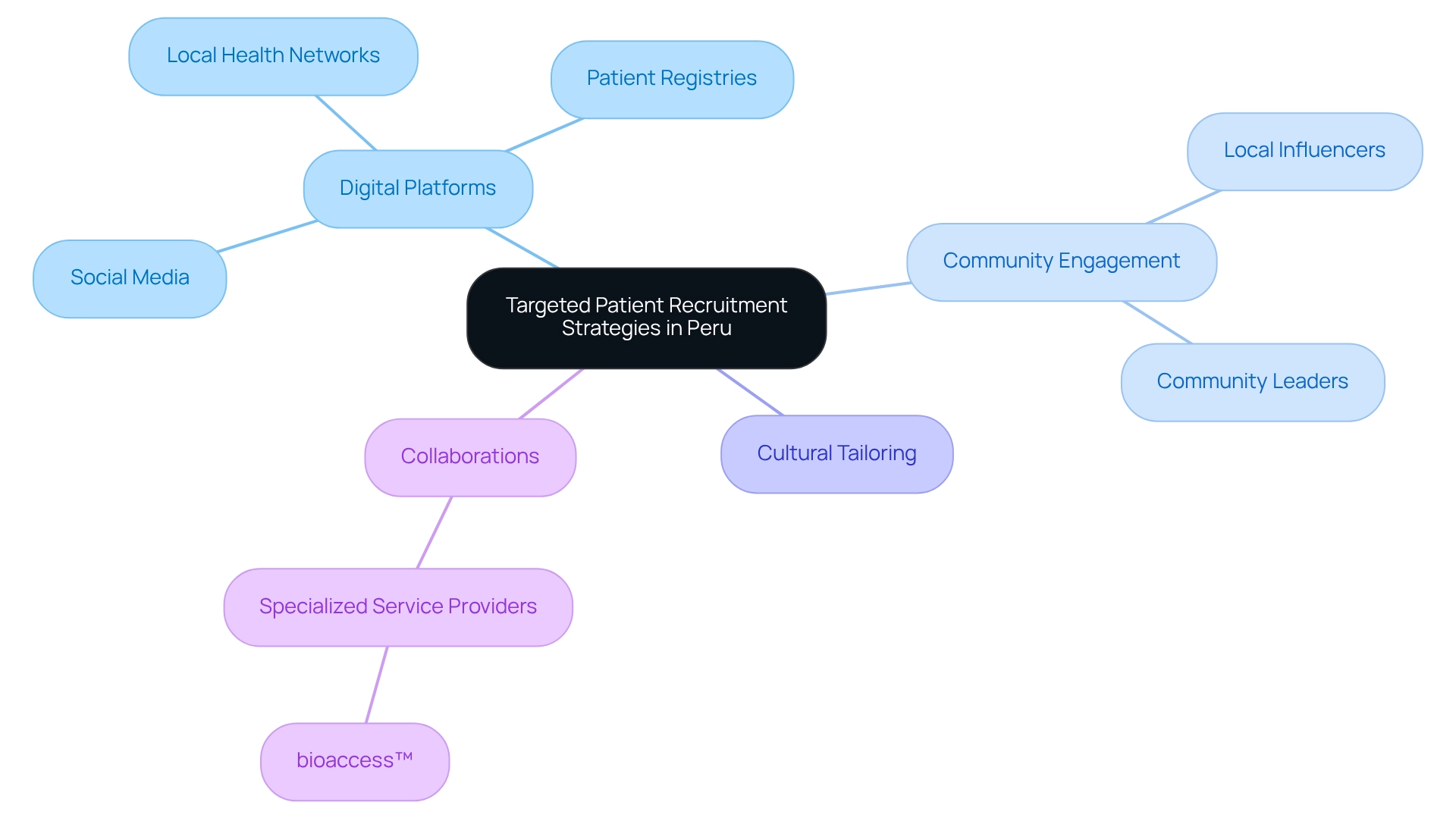
Implementing Targeted Patient Recruitment Strategies in Peru
Executing focused patient enrollment tactics is crucial for the success of research studies in Peru. By leveraging digital platforms such as social media, patient registries, and local health networks, outreach efforts can be significantly amplified. Engaging local influencers and community leaders not only fosters trust but also encourages participation, which is vital in a diverse cultural landscape. Tailoring recruitment messages to align with the cultural values and health concerns of the target population further enhances enrollment rates.
In 2025, the effectiveness of these strategies is underscored by the projected 7.7 percent compound annual growth rate (CAGR) for the contract research outsourcing market, highlighting the increasing reliance on specialized recruitment methods. This expansion signifies a transition toward more creative and effective hiring methods, essential for addressing the needs of research studies in Peru. Collaborations with specialized service providers, like bioaccess™, exemplify how patient recruitment can be simplified, ensuring research projects achieve enrollment objectives effectively. As bioaccess® states, "There are many benefits on teaming up with us such as gaining instant credibility, access to an extensive database of patients that already trust us, years of expertise and most importantly, we help making patient’s lives better." This emphasizes the value of collaboration in overcoming recruitment challenges.
Furthermore, the influence of social networks on research enrollment in Latin America is significant; they serve as a vital resource for contacting potential participants and disseminating information about current studies. By utilizing these digital platforms effectively, healthcare technology firms can drive medtech innovation through Peruvian research and enhance their recruitment outcomes in Peru. The partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a premier location for clinical studies further demonstrates the beneficial influence of local knowledge and collaborations on the success of clinical research efforts.
Utilizing Early-Feasibility Studies to Validate Medtech Innovations
Early-feasibility studies (EFS) play a crucial role in validating medical technology innovations, offering essential preliminary data on safety and functionality. Conducting EFS in Peru, with the support of bioaccess®, empowers developers to assess their products under real-world conditions, facilitating necessary adjustments before larger pivotal trials, highlighting the importance of Medtech innovation through Peruvian research. This proactive strategy aids in identifying potential challenges early, significantly mitigating the risk of costly failures later in the development process.
Moreover, collaborating with local regulatory bodies, such as INVIMA, during this phase can streamline future approval processes, thereby enhancing the overall efficiency of bringing medical devices to market. As Latin America emerges as a center for medical devices, the emphasis on EFS, bolstered by bioaccess®'s expertise in managing a diverse range of studies—including:
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
is poised to foster Medtech innovation through Peruvian research, generating transformative impacts on patient care and the broader medical technology landscape. The bioaccess® team, armed with over 20 years of experience in medical technology, is exceptionally equipped to navigate these complexities. Looking forward, the future of EFS appears promising, driven by technological advancements and a commitment to patient-centric methodologies.

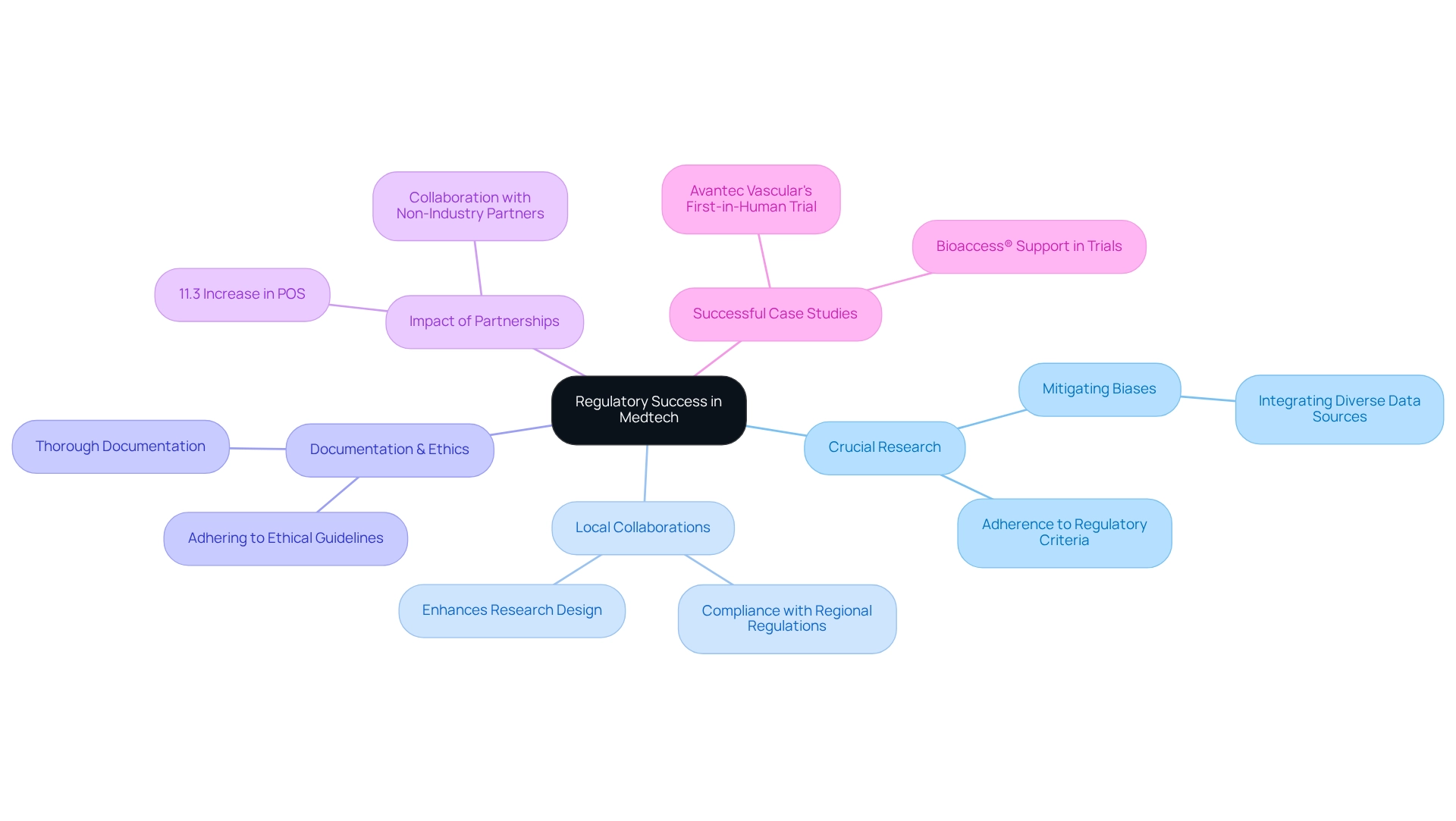
Conducting Pivotal Studies for Regulatory Success in Medtech
Conducting crucial research is essential for obtaining regulatory approval for Medtech devices. These analyses must be meticulously designed to conform to the stringent criteria set by regulatory bodies. In Peru, maintaining thorough documentation and adhering to ethical guidelines are vital to facilitate the approval process for Medtech innovation through Peruvian research.
Collaborating with local specialists not only enhances the research design and ensures compliance with regional regulations but also facilitates Medtech innovation through Peruvian research, significantly increasing the likelihood of successful outcomes. Notably, research indicates that engaging non-industry partners can lead to an 11.3 percentage point increase in the likelihood of success (POS) for drug development projects, underscoring the critical role of collaboration in achieving regulatory milestones.
Furthermore, understanding the nuances of drug development success rates is crucial for crafting effective pivotal research. Integrating diverse data sources can also mitigate biases in research reporting, thereby enhancing the reliability of investigations conducted in Peru, contributing to Medtech innovation through Peruvian research.
Successful pivotal studies, including Avantec Vascular's first-in-human trial of an innovative vascular device with bioaccess™ support, demonstrate that Medtech innovation through Peruvian research can effectively satisfy both local and international regulatory requirements. This paves the way for timely market entry and improved patient access to groundbreaking medical technologies.
As Andrew W. Lo emphasizes, teamwork and knowledge are essential in navigating the complexities of Medtech research, making organizations like bioaccess® integral in supporting trials across Latin America. Addressing the life science innovation gap between the US, Europe, and Latin America is imperative for ensuring that patients in Latin America gain faster and easier access to transformative medical technologies.
Ensuring Long-Term Success with Post-Market Clinical Follow-Up Studies
Post-market clinical follow-up (PMCF) evaluations are essential for ensuring the long-term safety and effectiveness of medical devices. These analyses provide vital information on device performance in real-world settings, enabling the detection of potential issues that may arise after market launch. In Peru, the implementation of PMCF research significantly enhances the credibility of Medtech firms, which is vital for driving Medtech innovation through Peruvian research and fostering trust among healthcare providers and patients alike. The consistent gathering and examination of information from these evaluations are crucial for continuous product enhancement and compliance with regulatory standards.
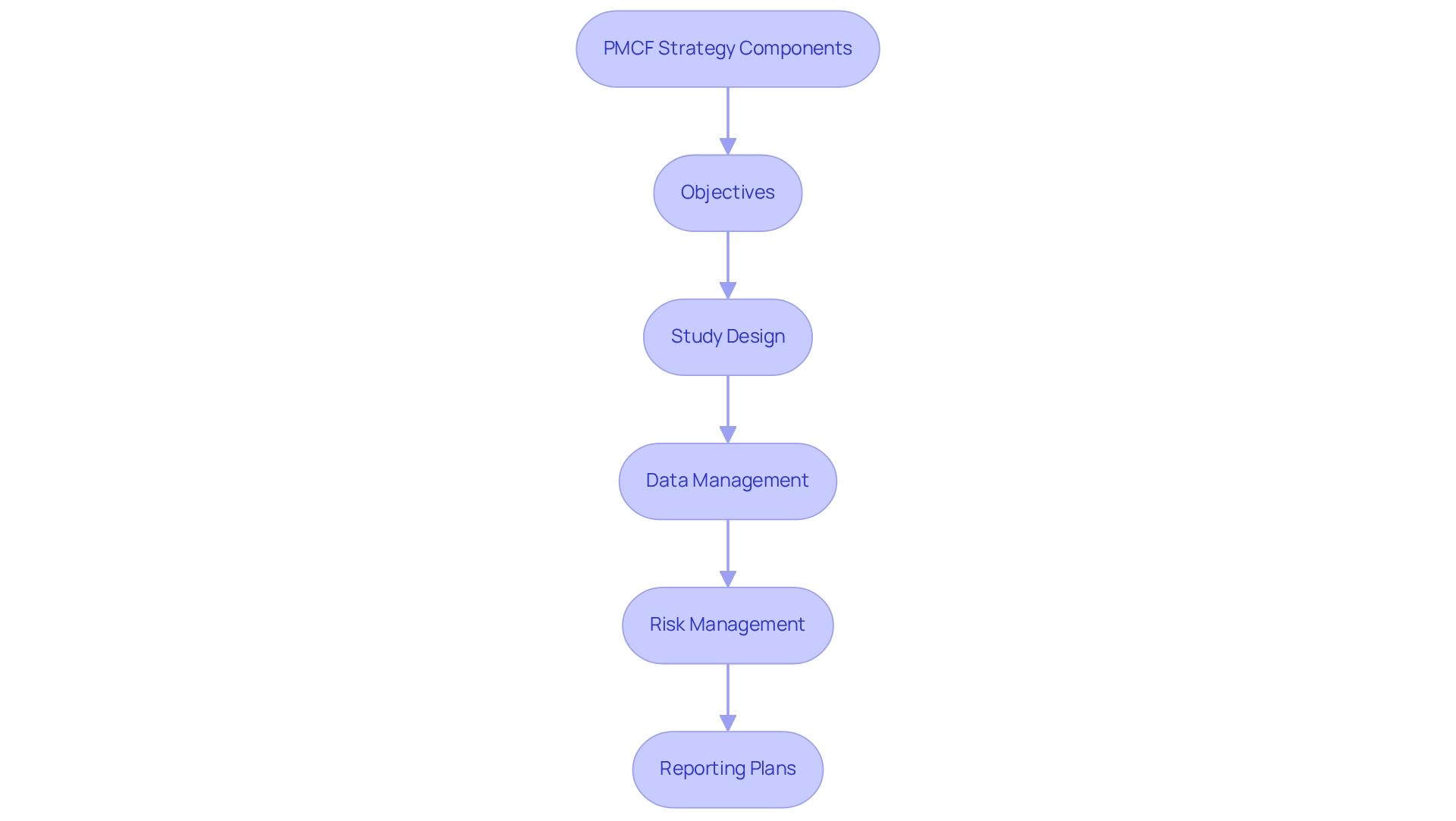
With the biologics market projected to grow at a compound annual growth rate of 15% until 2027, the demand for innovative PMCF strategies is more pressing than ever, particularly in addressing the unique challenges presented by the new generation of monoclonal antibodies (mAbs). A comprehensive PMCF strategy should encompass the following elements:
- Objectives
- Study design
- Data management
- Risk management
- Reporting plans
This ensures thorough evaluation and documentation of medical device safety. As emphasized by industry leaders, including insights from Steve Garchow, adaptability and local expertise are essential in navigating the complexities of the Latin American medical technology landscape.
Furthermore, bioaccess® is committed to ensuring information security and client trust throughout the clinical trial process, addressing any concerns through established grievance and data protection procedures. As Charles Darwin wisely remarked, "It is not the strongest of the species that survives, nor the most intelligent, but the one most responsive to change," underscoring the necessity for adaptability in the medical technology sector.
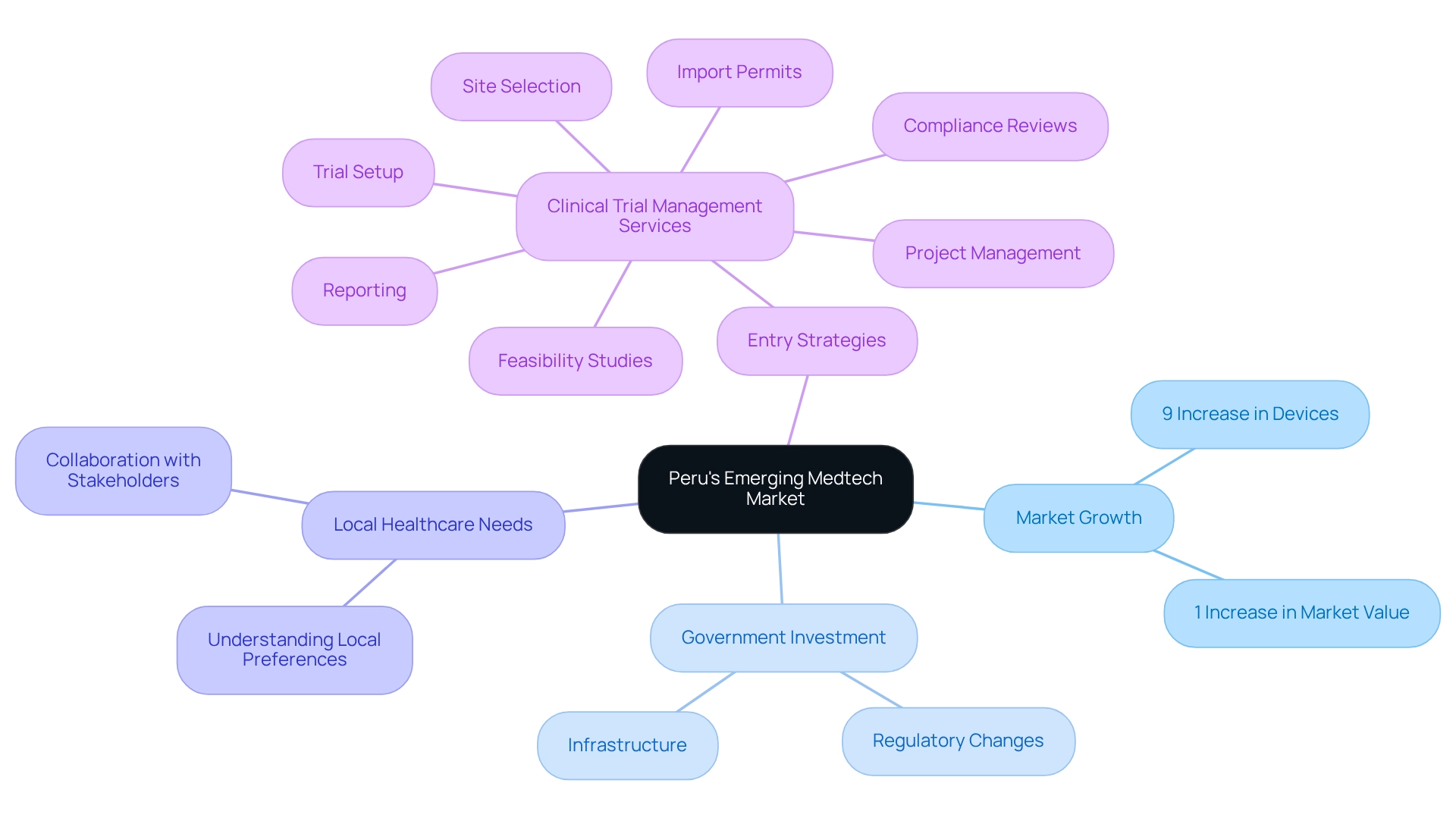
Exploring Peru's Emerging Market for Medtech Innovations
Peru's medical technology market is witnessing significant growth, driven by increasing healthcare demands and a burgeoning middle class. Recent statistics reveal a 9% uptick in the quantity of medical devices and a 1% increase in their market value, underscoring a robust demand for innovative solutions. The Peruvian government is actively investing in healthcare infrastructure, which is crucial for fostering medtech innovation through Peruvian research. This investment, coupled with favorable regulatory changes, positions Peru as an attractive destination for medical technology companies.
To effectively navigate this emerging market, firms must prioritize a deep understanding of local healthcare needs and preferences. Collaborating with local stakeholders can enhance market entry strategies, ensuring that innovations are tailored to address the specific challenges faced by the healthcare system. Comprehensive clinical trial management services—including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting—are essential for medical technology firms seeking to establish a foothold in Peru. These services not only streamline the research process but also contribute to job creation and economic growth, ultimately improving healthcare outcomes.
Insights from Global Health Intelligence's 2017 data portrait of the medical devices market in Latin America provide critical context for stakeholders, enabling informed decision-making. This data highlights the evolving environment and the opportunities for Medtech innovation through Peruvian research to address local needs.
As Julio G. Martinez-Clark, CEO, noted, "Peru is now removing temporary beds from service as the number of cases decreases," illustrating the dynamic shifts in healthcare demands. As the healthcare landscape evolves, medical technology firms that align their strategies with medtech innovation through Peruvian research will discover substantial growth opportunities in Peru. By addressing local healthcare demands and leveraging the country's investment in infrastructure, they can drive successful innovations that significantly enhance patient care.
Fostering Collaboration Between Medtech Companies and Local Research Institutions
Partnerships between Medtech firms and regional research organizations are essential for fostering medtech innovation through Peruvian research in Latin America. A significant instance is the collaboration between bioaccess™ and Caribbean Health Group, revealed on March 29, 2019, which seeks to establish Barranquilla as a prominent location for research trials in the region. This initiative, backed by Colombia's Minister of Health, highlights the significance of strategic alliances in boosting research capabilities and enhancing the quality of medical trials.
These collaborations not only promote knowledge exchange but also offer access to invaluable resources, including specialized expertise, diverse patient populations, and innovative research methodologies. For instance, successful collaborations have been crucial in enhancing research capabilities, contributing to Medtech innovation through Peruvian research, with over a 50% reduction in recruitment time and 95% retention rates. Moreover, partnerships with academic institutions strengthen the research ecosystem and expedite the development and commercialization of new medical technologies. Statistics show that successful teamwork can result in higher-quality medical studies, ultimately benefiting millions of patients.
As the MedTech industry navigates challenges in identifying high-growth areas, such as the need for innovation in cardiovascular therapeutics, leveraging local expertise through these partnerships presents a promising avenue for innovation and success. Julio Martinez-Clark, CEO of bioaccess, exemplifies this advocacy for Medtech clinical research in Latin America, focusing on operationalizing clinical trials and mentoring startups to promote regional opportunities.
Conclusion
The Medtech landscape in Peru is evolving rapidly, driven by innovative clinical research that positions the country as a burgeoning hub for medical device development. Central to this transformation is bioaccess®, which, with over 15 years of experience, plays a pivotal role in supporting Medtech startups through tailored clinical research services. By adeptly navigating the complexities of clinical trials, including regulatory compliance and strategic patient recruitment, bioaccess® is not only accelerating the development of medical technologies but also enhancing healthcare outcomes across the region.
Collaboration emerges as a cornerstone of success in this dynamic environment. Engaging local stakeholders and incorporating cultural insights are crucial for designing clinical trials that resonate with the target populations. This approach not only improves patient recruitment and retention but also fosters trust and compliance, ultimately leading to more effective studies. As highlighted throughout the article, the integration of local expertise and innovative strategies is essential for overcoming the unique challenges faced in the Peruvian healthcare system.
Looking ahead, the opportunities for Medtech innovation in Peru are substantial. The government's commitment to improving healthcare infrastructure, coupled with a favorable regulatory landscape, is creating a fertile ground for growth. Medtech companies that leverage these insights and prioritize collaboration with local entities stand to gain a significant competitive advantage. By aligning their strategies with the evolving needs of the healthcare market, they can drive meaningful advancements that enhance patient care and contribute to economic growth in the region.
Frequently Asked Questions
What services does bioaccess® provide for medical technology startups?
bioaccess® offers essential services that accelerate the development of medical devices, including feasibility and selection of research sites, investigator selection, regulatory compliance, project management, and detailed reporting.
How does bioaccess® help startups navigate research processes?
With over 15 years of experience, bioaccess® empowers medical technology startups to effectively tackle challenges such as regulatory hurdles and recruitment issues through a tailored approach and comprehensive methodology.
What is the significance of Peru's regulatory framework in medical technology studies?
Peru's regulatory framework, overseen by the National Institute of Health (INS), is crucial for the authorization and supervision of clinical research, ensuring that medical technology firms comply with ethical approvals, safety reporting, and documentation.
What recent changes have been made to Peru's regulatory approval process?
Recent updates for 2025 have streamlined the approval process, significantly reducing the average clinical study approval duration and making it more accessible for international companies to conduct research in Peru.
What role do local stakeholders play in successful medical studies in Peru?
Engaging local stakeholders, including healthcare professionals and community organizations, enhances patient recruitment and retention rates, ensuring studies are culturally relevant and tailored to community needs.
How does bioaccess® contribute to improving patient recruitment strategies?
bioaccess® emphasizes building robust relationships with local entities and leveraging local insights, which can significantly enhance enrollment success rates in clinical trials.
What is the impact of the COVID-19 pandemic on research participant enrollment?
The pandemic initially slowed participant enrollment but also accelerated innovative study designs and creative recruitment strategies, demonstrating adaptability in the field.
What are some of the comprehensive services offered by bioaccess® for research initiatives?
bioaccess® provides services such as feasibility assessments, site selection, compliance evaluations, research preparation, import permits, project management, and reporting, all crucial for successful research initiatives.




