Overview
This article presents eight compliance strategies that are pivotal for enhancing the success of clinical trials in Peru. It underscores the necessity of understanding local regulations and engaging local stakeholders, alongside preparing essential documentation and leveraging technology. These strategies collectively contribute to improved recruitment, adherence, and overall efficacy of research studies, highlighting their critical role in the clinical research landscape.
Introduction
Navigating the intricate landscape of clinical trials in Peru presents a unique set of challenges and opportunities for Medtech companies. As regulatory frameworks evolve and the demand for ethical compliance reaches unprecedented levels, organizations must adapt their strategies to ensure successful outcomes. bioaccess® emerges as a pivotal ally, armed with over two decades of expertise in guiding sponsors through the complexities of regulatory adherence, from trial setup to post-market evaluations.
By understanding local regulations, engaging with community stakeholders, and leveraging technology, companies can enhance their trial processes while fostering trust and transparency. This article delves into essential strategies that Medtech firms can implement to:
- Streamline compliance
- Maximize recruitment
- Ultimately drive innovation in the Peruvian healthcare landscape.
bioaccess®: Streamline Compliance with Expert Guidance for Trials in Peru
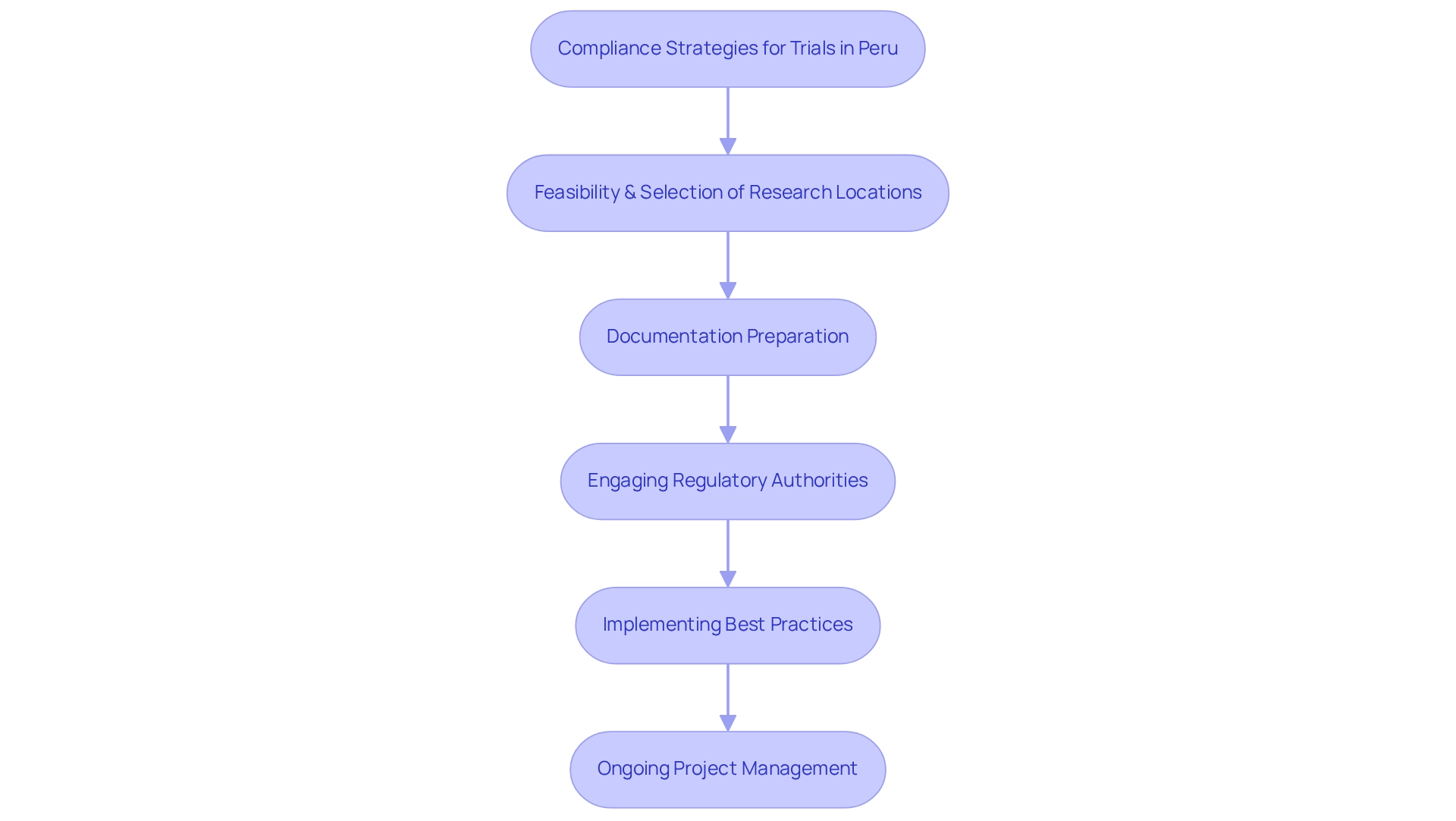
bioaccess® is dedicated to assisting Medtech firms in navigating the intricate compliance landscape in Peru by leveraging over 20 years of industry expertise to provide compliance strategies for trials in Peru. These compliance strategies for trials in Peru are designed to ensure adherence to local regulations, significantly enhancing the likelihood of success.
The professional team at bioaccess® plays a crucial role in the feasibility and selection of research locations and principal investigators, preparing necessary documentation, engaging with regulatory authorities, and implementing best practices throughout the study lifecycle. This comprehensive approach simplifies regulatory processes for sponsors, encompassing trial setup, import permits, and ongoing project management and reporting.
Moreover, with the National Registry of Chemical Substances (RENASQ) integrating information on hazardous chemicals, compliance strategies for trials in Peru have become increasingly critical for Medtech companies operating in the region. As Adrian Ebner, a principal investigator involved in over 70 first-in-human studies, asserts, 'Expert guidance is essential for navigating the compliance landscape and ensuring successful outcomes.'
By examining Colombia's recent expansion in research studies, where a consistent oversight framework has yielded notable advancements in medical technology, Medtech firms can navigate the oversight landscape with confidence, ultimately enhancing the productivity and efficacy of their research studies in Peru.
Understand Peru's Regulatory Framework: A Foundation for Compliance
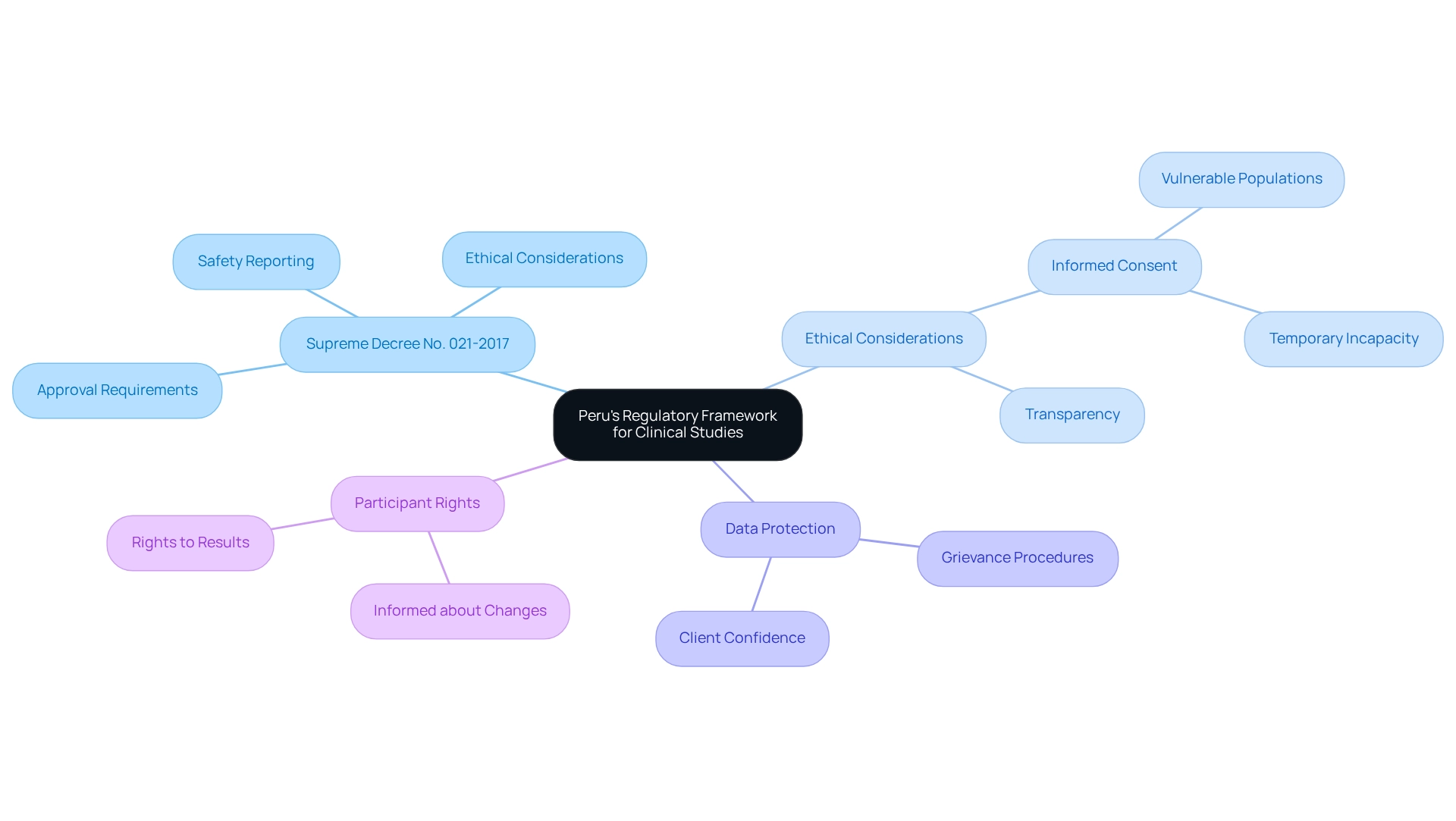
Peru's regulatory framework for clinical studies is primarily overseen by the National Institute of Health (INS), anchored by key legislation, notably Supreme Decree No. 021-2017. This decree outlines essential requirements for the approval of testing, ethical considerations, and safety reporting. It is imperative for sponsors to thoroughly understand these regulations. A comprehensive grasp of the legal landscape not only helps in steering clear of common pitfalls but also ensures that compliance strategies for trials in Peru are implemented in an ethical and compliant manner.
At bioaccess®, we are dedicated to ensuring information security and client confidence throughout the research process. Our grievance and data protection procedures are meticulously designed to address client concerns while adhering to regulations and promoting transparency. This reinforces our commitment to ethical practices in medical device trials. Clients can reach out to our Grievance Officer at IMH ASSETS CORP (doing business as "bioaccess®"), located at 1200 Brickell Avenue, Suite 1950 #1034, or via email at info@bioaccessla.com for any inquiries or concerns regarding their data protection rights.
Supreme Decree No. 021-2017 has undergone updates that further enhance regulations, improving the integrity of medical research in the country. Notably, it mandates that participants must be informed about any changes, progress, and results of the research, aligning with international standards for participant rights. This requirement is crucial for maintaining transparency and trust in the research process.
Moreover, the regulatory framework emphasizes the protection of vulnerable populations, particularly in sensitive areas such as mental health. A notable case study illustrates how regulations ensure that individuals with mental health challenges are treated with dignity and respect, safeguarding their rights to informed consent, even when they may be temporarily incapacitated. The absence of information about the waiver of consent in research studies further underscores the significance of informed consent in adherence strategies.
Grasping these regulations involves more than mere compliance; it emphasizes building trust and guaranteeing ethical practices in research, particularly through compliance strategies for trials in Peru. The DIIS serves as a valuable resource for sponsors, providing contact details for inquiries related to research studies, which aids in navigating this intricate environment. By effectively maneuvering through Peru's research regulations, sponsors can enhance the success of their studies while contributing to the advancement of medical technology in the region.
Prepare Essential Documentation: Key to Regulatory Compliance
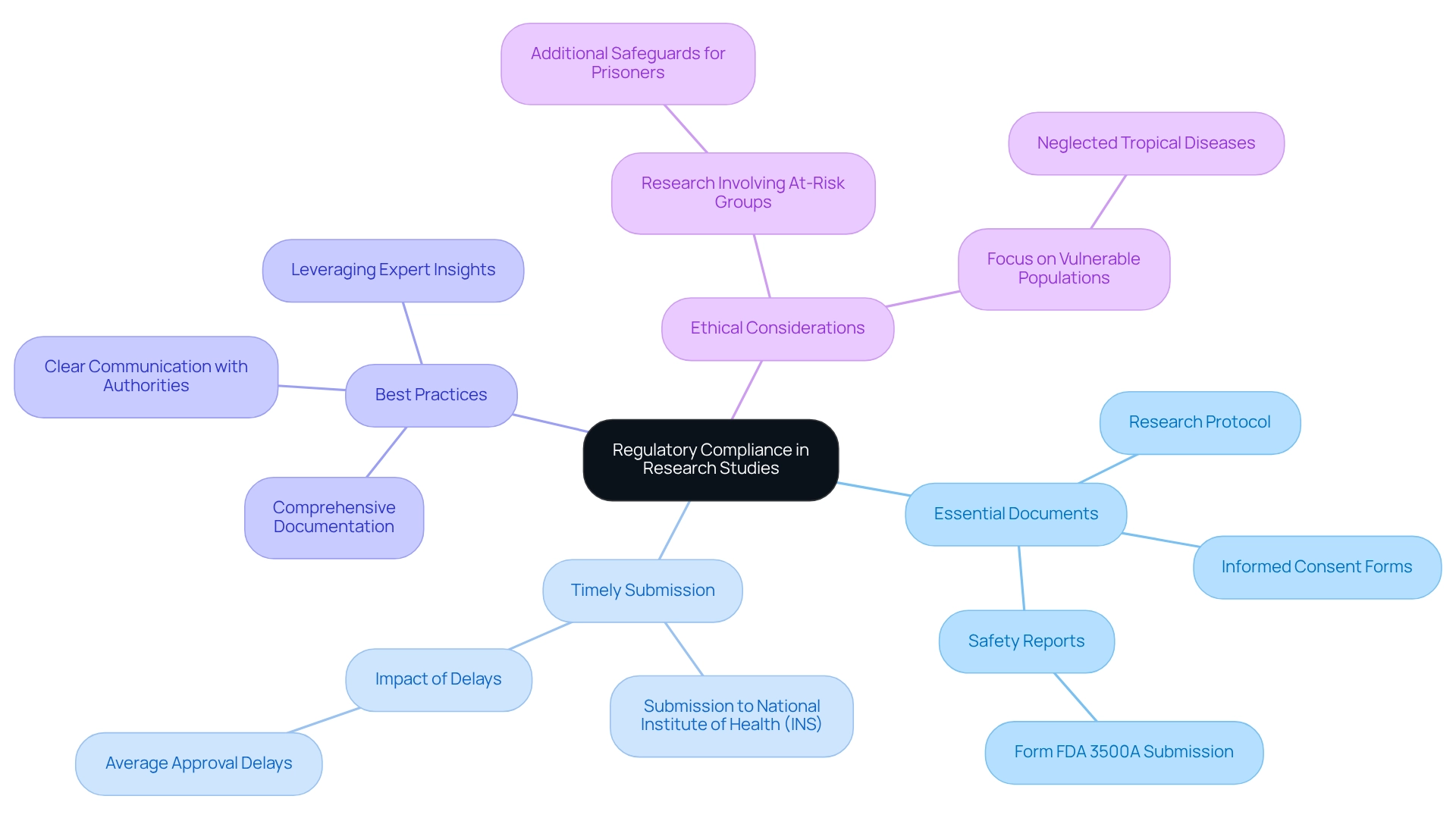
Effective preparation of essential paperwork is vital for ensuring compliance with regulations in Peru's research studies. These essential documents—comprising the research protocol, informed consent forms, and safety reports—must adhere to specific regulatory standards. The clinical study protocol outlines the study's objectives, design, and methodology, while informed consent forms guarantee that participants are fully informed of the study's risks and benefits. Safety reports, provided in a narrative format on Form FDA 3500A, are crucial for overseeing participant safety during the study.
In Peru, timely submission of these documents to the National Institute of Health (INS) is imperative to prevent delays in study approval. bioaccess® plays a pivotal role in assisting sponsors with the compilation and review of these documents, ensuring completeness and compliance. This proactive approach significantly reduces the likelihood of approval holdups, which can average several months based on the quality of the submitted paperwork.
Best practices for preparing research protocols in Peru include comprehensive documentation of all study procedures and strict adherence to local guidelines. Additionally, maintaining clear communication with oversight authorities and leveraging expert insights can enhance the quality of submissions. Notably, guidelines for research involving at-risk groups, such as prisoners, necessitate extra protections to safeguard their rights and welfare, underscoring the importance of ethical considerations in study preparations. Furthermore, Gabriela Minaya from the National Institute of Health of Peru has highlighted a troubling trend of restricted approved studies focusing on neglected tropical diseases, which emphasizes the need for increased attention to health issues affecting vulnerable populations. By adopting these strategies and understanding the regulatory landscape—including the impacts of COVID-19 on administrative procedures—sponsors can navigate the complexities of regulatory requirements more efficiently, ultimately facilitating smoother testing processes and advancing medical innovations in the field.
Engage Local Stakeholders: Boost Recruitment and Compliance
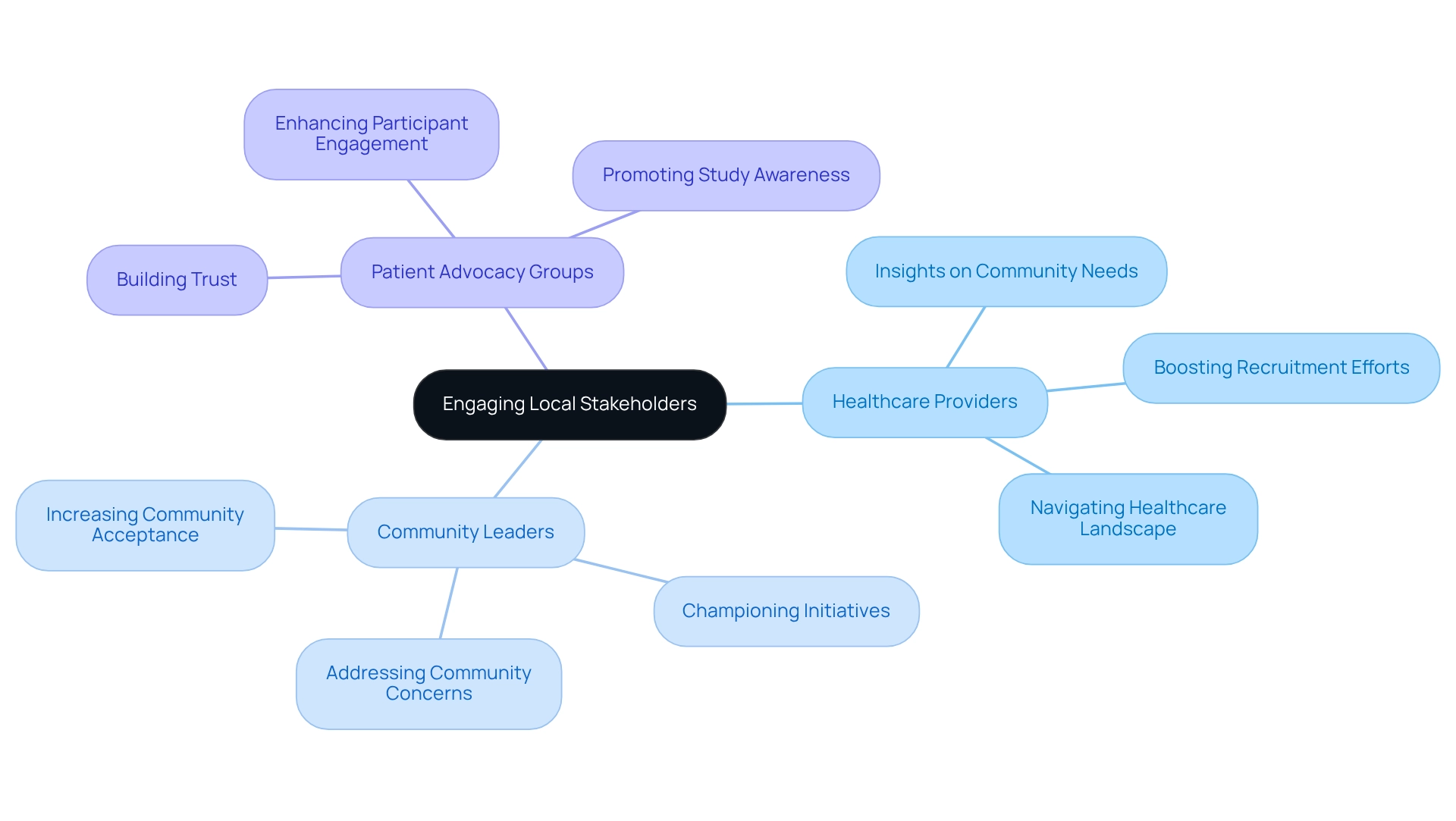
Involving local stakeholders—such as healthcare providers, community leaders, and patient advocacy groups—is essential for enhancing recruitment and ensuring adherence in clinical studies. These stakeholders offer vital insights into community needs and preferences, significantly informing study design and recruitment strategies. Establishing strong connections with local organizations fosters trust among participants, leading to improved retention rates and, ultimately, more successful study outcomes.
In Peru, where cultural nuances and community dynamics are paramount, employing compliance strategies for trials in Peru by leveraging the expertise of local healthcare providers can substantially boost recruitment efforts. Effective strategies include collaborating with community leaders who can champion the initiative and address any concerns, thereby increasing community acceptance.
Statistics reveal that studies with robust local stakeholder engagement experience a notable uptick in recruitment success, highlighting the impact of community involvement on clinical study outcomes. For instance, a recent case study illustrated that a study in Peru, which actively engaged local healthcare providers and community leaders, achieved a 30% higher recruitment rate compared to studies that did not.
By prioritizing these relationships, sponsors can adeptly navigate the complexities of the Peruvian healthcare landscape, ensuring that their compliance strategies for trials in Peru not only meet regulatory standards but also resonate with the communities they aim to serve. Furthermore, the partnership between bioaccess™ and Caribbean Health Group to position Barranquilla as a premier research study location in Latin America exemplifies how strategic alliances can enhance study success. With over 15 years of experience in the Medtech sector, bioaccess® is uniquely positioned to cultivate these essential connections and oversee various clinical studies, ensuring that clinical research positively influences local economies through job creation and healthcare improvement.
Incorporate Cultural Insights: Enhance Trial Design and Compliance
Integrating cultural insights into study design is essential for ensuring that research remains relevant and respectful to local communities. This integration involves a deep understanding of local health beliefs, practices, and preferences. By tailoring study protocols to align with cultural values, sponsors can enhance participant involvement and adherence, leading to more effective recruitment strategies and improved study outcomes.
Additionally, bioaccess offers a comprehensive range of research study management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
These services not only facilitate smoother test execution but also contribute to the overall success of research studies in Peru, emphasizing the need for compliance strategies for trials in Peru to promote international cooperation and economic development within local healthcare systems.
To maximize the impact of your experiments, consider how you can effectively incorporate cultural insights into your study designs.
Implement Targeted Patient Recruitment Strategies: Ensure Compliance and Success
Applying focused patient recruitment strategies is crucial for guaranteeing adherence and achieving the goals of research studies. In 2025, leveraging digital marketing and community outreach, along with forming partnerships with local healthcare providers, can significantly enhance recruitment efforts. By utilizing customized methods to identify and engage potential participants, sponsors can improve enrollment rates and ensure that studies accurately reflect the demographics of the target population. This is vital, as 90% of research studies encounter delays due to low enrollment rates and inefficient processes, underscoring the necessity for effective recruitment strategies.
Successful digital marketing techniques, such as leveraging social media platforms and targeted online advertising, have demonstrated success in reaching diverse populations. Understanding patient preferences is essential; research indicates that non-white individuals prioritize communication with the hospital or company overseeing the research more than their white counterparts. This highlights the need for culturally sensitive outreach strategies that resonate with various demographic groups.
Case studies illustrate that seamless communication between sponsors and research sites can bridge gaps in patient recruitment. For instance, the partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a leading location for medical research in Latin America, supported by Colombia's Minister of Health. This enhanced collaboration has led to improved enrollment outcomes, ultimately resulting in more reliable data and safer, more effective products for the entire population. Additionally, bioaccess®'s expertise in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF) underscores the significance of thorough management services in achieving successful outcomes.
To implement these targeted recruitment strategies effectively, Medtech companies should prioritize building strong relationships with local communities and continuously adapt their outreach efforts based on demographic insights. By concentrating on compliance strategies for trials in Peru, Medtech firms can navigate the complexities of medical studies and achieve successful results.
Maintain Compliance Throughout the Trial Lifecycle: Best Practices
Ensuring adherence throughout the clinical trial lifecycle necessitates the implementation of compliance strategies for trials in Peru, which requires a proactive and structured approach to monitoring and management. Key best practices include:
- Regular Staff Training: Continuous education on regulatory requirements is crucial. Bioaccess offers training programs that are regularly updated to align with the latest regulations and standards, fostering a knowledgeable workforce.
- Internal Audits: Conducting regular internal audits aids in detecting potential regulatory issues before they escalate. Bioaccess assists in these audits to assess adherence to protocols and regulatory guidelines, ensuring that all aspects of the trial are scrutinized.
- Clear Communication Channels: Establishing transparent communication pathways for reporting regulatory concerns is essential. Bioaccess encourages staff to voice issues without fear of repercussions, promoting a culture of accountability.
- Monitoring Adherence: Implementing centralized monitoring systems can significantly enhance oversight. Bioaccess assists organizations in creating these systems, ensuring that adherence is a collective duty at all levels.
- Impact of Staff Training: Research indicates that effective training programs can lead to a significant enhancement in adherence rates. For example, an analysis of 158 randomized controlled studies (RCTs) showed that only 21 investigations (13%) used techniques to address adherence, highlighting the necessity for improved education and awareness.
- Expert Insights: Compliance officers emphasize the importance of integrating statistical methods to adjust for compliance issues in clinical research. As Junichi Sakamoto noted, "Experience from real tests as well as extensive simulation studies have shown that a statistical data quality assessment based on the principles outlined above is quite effective at detecting data errors." This method not only improves data integrity but also enhances the overall quality of the results.
- Best Practices for 2025: As the environment of clinical studies changes, embracing novel adherence strategies will be essential. Bioaccess utilizes technology for real-time monitoring and data quality evaluations, greatly enhancing regulatory oversight.
- Comprehensive Clinical Study Management Services: Bioaccess provides a variety of services that assist with adherence, including feasibility studies, site selection, review processes, study setup, import permits, project management, and reporting. These services guarantee that assessments comply with the highest ethical and legal standards.
- Statistics on Adherence Monitoring: Significantly, variability in research costs can surpass 100-fold among the 138 studies surveyed, emphasizing the financial consequences of adherence failures.
By applying compliance strategies for trials in Peru, sponsors can reduce risks and ensure that clinical trials meet the highest ethical and legal standards, ultimately improving the success of their research initiatives.
Design Post-Market Studies: Ensure Continued Compliance and Evaluation
Creating post-market studies is essential for upholding regulations and evaluating the long-term safety and effectiveness of medical devices. These studies must align with regulatory requirements and incorporate robust mechanisms for collecting data on adverse events and device performance. By conducting thorough post-market assessments, sponsors not only demonstrate continuous adherence but also strengthen the evidence foundation backing their products.
Expert insights emphasize that a well-organized post-market study can significantly enhance the understanding of a device's actual performance in the field. Successful strategies often involve continuous monitoring and adaptive methodologies that respond to emerging data. A notable case study is the collaboration between CVRx and NAMSA, a prominent research organization, which underscored the necessity of obtaining safety and effectiveness evidence for FDA approval. This partnership facilitated a comprehensive assessment procedure, ultimately leading to the successful commercialization of life-saving technology for heart failure patients, which underscores the significance of compliance strategies for trials in Peru, as adherence rates for post-market studies are becoming increasingly vital.
As of 2025, the landscape for post-market evaluations has evolved, necessitating a focus on long-term safety assessments. Regulatory experts advocate for a proactive approach to these evaluations, highlighting their impact on medical device safety statistics. bioaccess® provides extensive management services for studies, including proficiency in post-market follow-up research (PMCF), ensuring that companies can navigate the intricacies of legal adherence effectively. By prioritizing post-market studies, companies can not only meet compliance requirements but also enhance the overall safety and effectiveness of their medical devices in the market.
Train Your Team: Foster a Culture of Compliance in Clinical Trials
Educating your group on regulatory necessities and best practices is essential for developing compliance strategies for trials in Peru and cultivating a culture of adherence in research studies. Regular training sessions should cover Good Clinical Practice (GCP), regulatory updates, and ethical considerations, specifically addressing the unique challenges of the region. By equipping all team members with the necessary knowledge and involvement, sponsors can significantly enhance adherence and elevate the overall quality of their research studies.
A recent survey revealed that 82% of participants at the NIHR Statistics Group annual meeting found the training materials relevant to their roles, highlighting the effectiveness of role-specific GCP training. This relevance is particularly crucial in Peru, where compliance strategies for trials necessitate a thorough understanding of local regulations and practices. Furthermore, effective training programs have garnered international recognition, fostering a global dialogue on the importance of customized GCP training for research teams.
bioaccess offers comprehensive research study management services—including feasibility assessments, site selection, regulatory reviews, study setup, import permits, project management, and reporting—that are vital in supporting these training initiatives. Each of these services plays a direct role in enhancing training by providing the essential framework and resources.
As Roy H. Williams aptly stated, "Training is not an expense, but an investment in human capital." This approach not only improves adherence rates but also ensures that teams, including statisticians crucial for maintaining study quality, are well-prepared to implement compliance strategies for trials in Peru while navigating the complexities of research.
Leverage Technology: Enhance Compliance Monitoring in Clinical Trials
Employing technology to enhance oversight in clinical studies is crucial for boosting both efficiency and precision. Electronic data capture (EDC) systems, compliance tracking software, and remote monitoring solutions enable real-time oversight of study activities, ensuring conformity to legal standards. The implementation of EDC systems incorporates built-in checks that enhance data quality, which is essential for maintaining participant safety and data integrity.
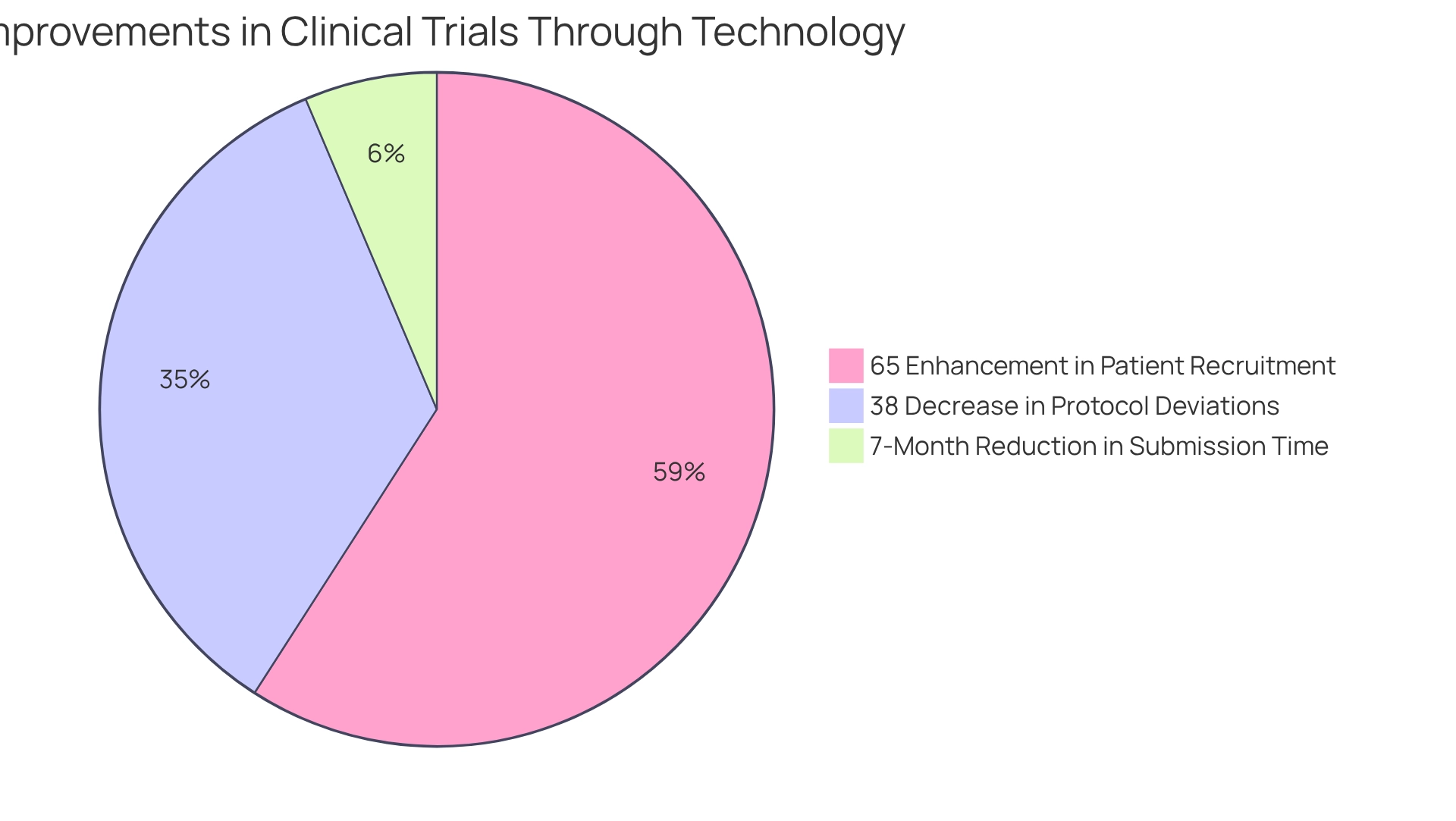
The adoption of EDC systems in Peru is a key aspect of compliance strategies for trials in Peru that has shown promising results. A notable case study involved a biotech firm specializing in rare diseases that leveraged a machine learning algorithm to optimize their data management process. This initiative resulted in:
- A 65% enhancement in patient recruitment through improved targeting
- A 38% decrease in protocol deviations
- A seven-month reduction in time to submission for approval
Statistics reveal that the worldwide market for AI in medical studies is expected to expand at a compound annual growth rate (CAGR) of 22.1% from 2023 to 2028, attaining $4.9 billion by 2028. This growth underscores the rising dependence on technology for oversight in clinical studies, indicating that sponsors who embrace these innovations will be better equipped to satisfy governance standards and improve study adherence rates.
Expert opinions emphasize the significance of incorporating advanced technologies into regulatory strategies. As Prof. Dr. Martin Samy noted, "In an accreditation process, there is emphasis on the efficacy of the IT infrastructure behind the medical practice." By utilizing EDC systems, sponsors can enhance compliance strategies for trials in Peru while fulfilling regulatory requirements and significantly improving compliance rates. Furthermore, with bioaccess's comprehensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies, the integration of these technologies can streamline data collection processes, reduce errors, and ultimately contribute to the successful advancement of medical devices in the market.
Conclusion
Navigating the complexities of clinical trials in Peru necessitates a strategic approach that underscores compliance, community engagement, and cultural sensitivity. Organizations such as bioaccess® are pivotal in guiding Medtech companies through this intricate landscape, ensuring adherence to local regulations while fostering innovation in the healthcare sector. By streamlining compliance processes, preparing essential documentation, and engaging local stakeholders, companies can significantly enhance their trial success rates and contribute positively to the advancement of medical technology.
A foundational understanding of Peru's regulatory framework is critical for compliance, while targeted patient recruitment strategies can markedly improve enrollment and retention. Moreover, the incorporation of technology in compliance monitoring not only enhances data quality but also accelerates trial timelines. By emphasizing the importance of training and maintaining open communication channels within teams, organizations foster a culture of compliance that is essential for overcoming the challenges of clinical trials.
Ultimately, by adopting these strategies and leveraging the expertise of partners like bioaccess®, Medtech companies can adeptly navigate the regulatory environment, drive innovation, and ensure that their clinical trials not only meet compliance standards but also resonate with the communities they aim to serve. As the landscape continues to evolve, a steadfast commitment to ethical practices and community engagement will be vital in shaping the future of clinical research in Peru.
Frequently Asked Questions
What is bioaccess® and its role in compliance for Medtech firms in Peru?
bioaccess® assists Medtech firms in navigating the compliance landscape in Peru by leveraging over 20 years of industry expertise to provide compliance strategies for trials, ensuring adherence to local regulations and enhancing the likelihood of success.
What does the compliance strategy for trials in Peru involve?
The compliance strategy includes feasibility and selection of research locations, preparation of necessary documentation, engagement with regulatory authorities, and implementation of best practices throughout the study lifecycle.
Who oversees the regulatory framework for clinical studies in Peru?
The regulatory framework for clinical studies in Peru is primarily overseen by the National Institute of Health (INS), guided by key legislation such as Supreme Decree No. 021-2017.
What are the key aspects of Supreme Decree No. 021-2017?
This decree outlines essential requirements for the approval of testing, ethical considerations, and safety reporting, which are crucial for sponsors to understand to avoid common pitfalls and ensure compliance.
How does bioaccess® ensure information security and client confidence?
bioaccess® has grievance and data protection procedures designed to address client concerns while adhering to regulations and promoting transparency, reinforcing their commitment to ethical practices in medical device trials.
What are the requirements regarding participant information in research studies?
Participants must be informed about any changes, progress, and results of the research, which aligns with international standards for participant rights and is essential for maintaining transparency and trust.
How does the regulatory framework protect vulnerable populations?
The framework emphasizes the treatment of vulnerable populations, particularly in sensitive areas like mental health, ensuring their rights to informed consent are respected, even if they may be temporarily incapacitated.
What is the importance of essential paperwork in compliance?
Essential documents such as research protocols, informed consent forms, and safety reports must adhere to specific regulatory standards to ensure compliance and participant safety during studies.
How can sponsors facilitate smoother testing processes in Peru?
By understanding the regulatory landscape, timely submission of essential documents, and maintaining clear communication with oversight authorities, sponsors can navigate complexities and enhance the success of their studies.
What challenges are currently faced in research studies in Peru?
There is a troubling trend of restricted approved studies focusing on neglected tropical diseases, highlighting the need for increased attention to health issues affecting vulnerable populations.




