Overview
The article addresses the challenges faced in designing clinical trials in Bolivia and suggests various strategies to effectively overcome these obstacles. It underscores the critical importance of local expertise, effective communication, and meticulous logistical planning. These elements are essential for navigating the regulatory complexities, recruiting participants, ensuring data integrity, and managing timelines effectively, all within the unique landscape of Bolivia. By leveraging these strategies, stakeholders can enhance the success of clinical trials in this region.
Introduction
In the realm of clinical research, conducting trials in diverse environments presents both opportunities and challenges. Bolivia, with its unique regulatory landscape and cultural nuances, necessitates a tailored approach to ensure the success of clinical studies. Bioaccess® emerges as a frontrunner, offering specialized solutions that adeptly navigate these complexities while enhancing the efficiency of trial execution.
From managing regulatory compliance to recruiting qualified participants and overcoming logistical hurdles, bioaccess® leverages over 15 years of Medtech experience to support innovative medical devices in reaching the market more effectively.
This article delves into the multifaceted strategies employed by bioaccess® to foster successful clinical trials in Bolivia, underscoring their unwavering commitment to quality, integrity, and local engagement.
bioaccess: Tailored Solutions for Clinical Trials in Bolivia
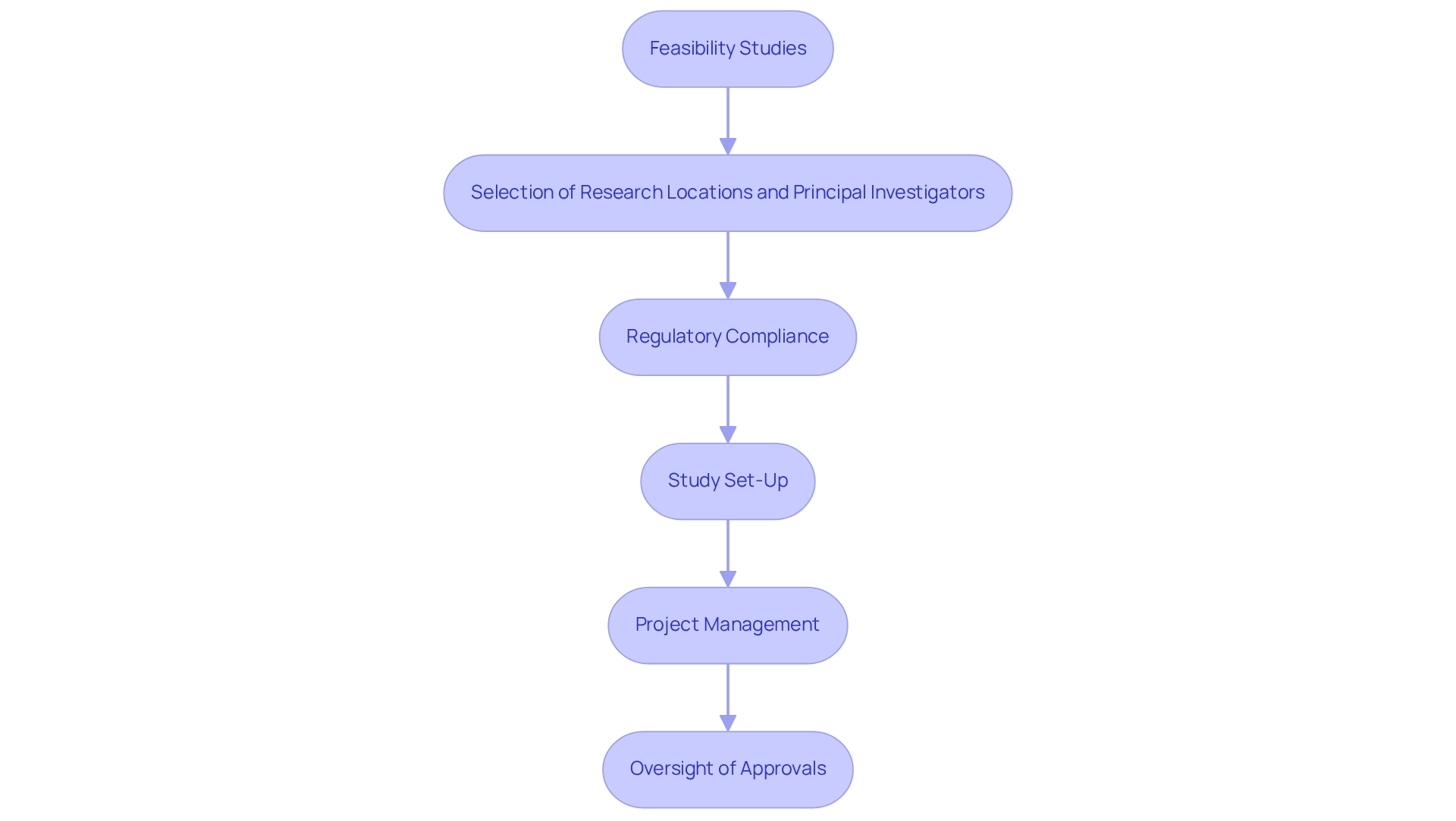
The company specializes in providing personalized research solutions that address the challenges in designing trials for Bolivia. With over 15 years of experience in the Medtech industry, the company offers a comprehensive range of services, including:
- Feasibility studies
- Selection of research locations and principal investigators
- Regulatory compliance
- Study set-up
- Project management
Their deep understanding of local regulations and cultural nuances allows them to streamline processes and enhance the efficacy of research trials. By overseeing approvals from ethics committees and health ministries, import permits, and the nationalization of investigational devices, as well as reporting on study status and adverse events, the organization ensures that innovative medical devices can enter the market more swiftly and effectively. This expertise positions bioaccess® as a leading CRO in Latin America, dedicated to supporting Medtech startups in navigating regulatory hurdles and financial constraints while advancing their research initiatives.
BOOK A MEETING.
Navigating Regulatory Complexities in Bolivia
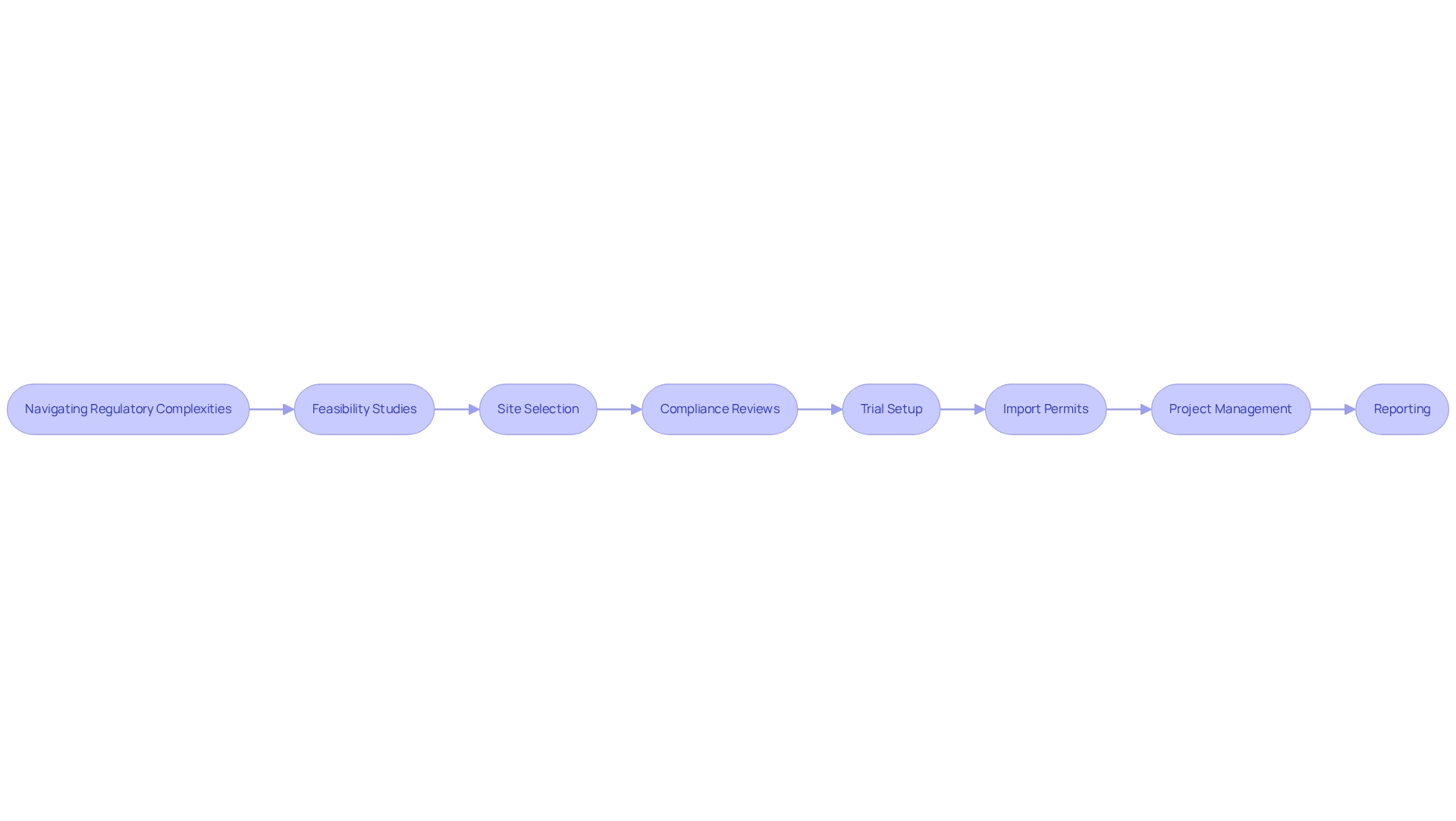
The regulatory landscape for clinical research in Bolivia presents challenges in designing trials for Bolivia that are unique and differ from other regions. Understanding the role of regional regulatory authorities, particularly the National Medicines Agency (ANM), along with their guidelines, is crucial for study sponsors. Engaging with local experts who possess in-depth knowledge of these regulations can facilitate smoother approvals and compliance.
bioaccess® provides a comprehensive suite of clinical trial management services, encompassing:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This ensures that all necessary documentation and ethical approvals are secured prior to the commencement of trials. Such a commitment to regulatory excellence not only streamlines the process but also fosters client trust through robust data protection measures.
For any inquiries related to data protection, clients are encouraged to contact our Grievance Officer, thereby ensuring transparency and adherence to applicable laws. This collaborative approach underscores the importance of partnership in addressing the challenges in designing trials for Bolivia.
Recruiting Qualified Participants in Clinical Trials
Recruiting qualified participants in Bolivia highlights the challenges in designing trials for Bolivia, which necessitates a multifaceted approach. Strategies include:
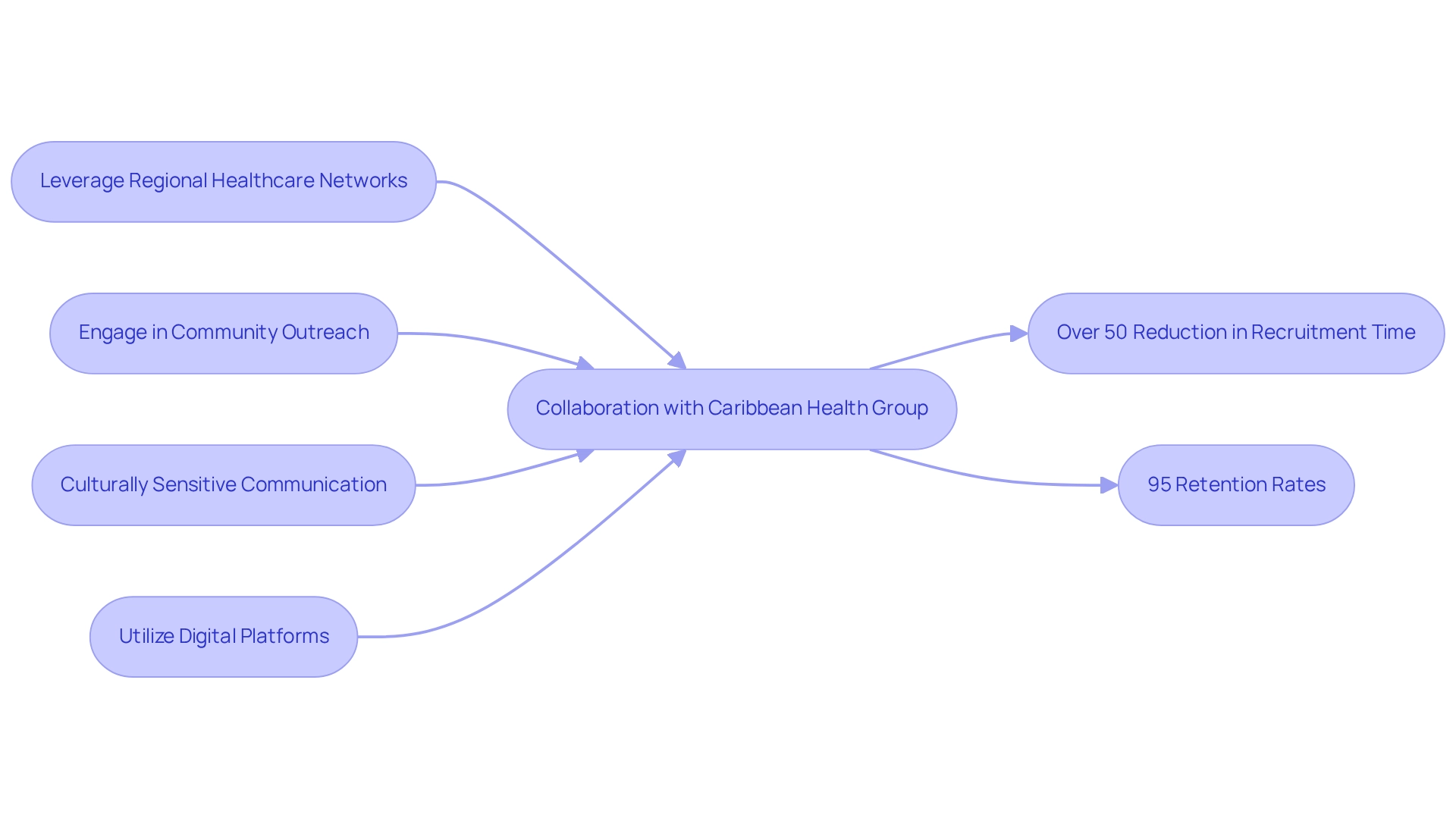
- Leveraging regional healthcare networks
- Engaging in community outreach
- Addressing the challenges in designing trials for Bolivia through culturally sensitive communication
Collaborating with local healthcare providers is essential to overcome the challenges in designing trials for Bolivia, as it fosters trust and enhances participant involvement. Furthermore, the company implements targeted recruitment efforts that resonate with the community to overcome the challenges in designing trials for Bolivia, ensuring a diverse and representative sample for research studies.
This approach is further strengthened by its partnership with Caribbean Health Group, which aims to position Barranquilla as a leading site for research studies in Latin America, supported by the involvement of Colombia's Minister of Health. This collaboration has notably achieved over a 50% reduction in recruitment time and 95% retention rates, underscoring its effectiveness.
Additionally, utilizing digital platforms for outreach broadens the reach and improves recruitment efficiency, ultimately contributing to the success of research studies and their positive impact on local economies. The company specializes in managing various types of research, including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
Overcoming Logistical Challenges in Remote Areas
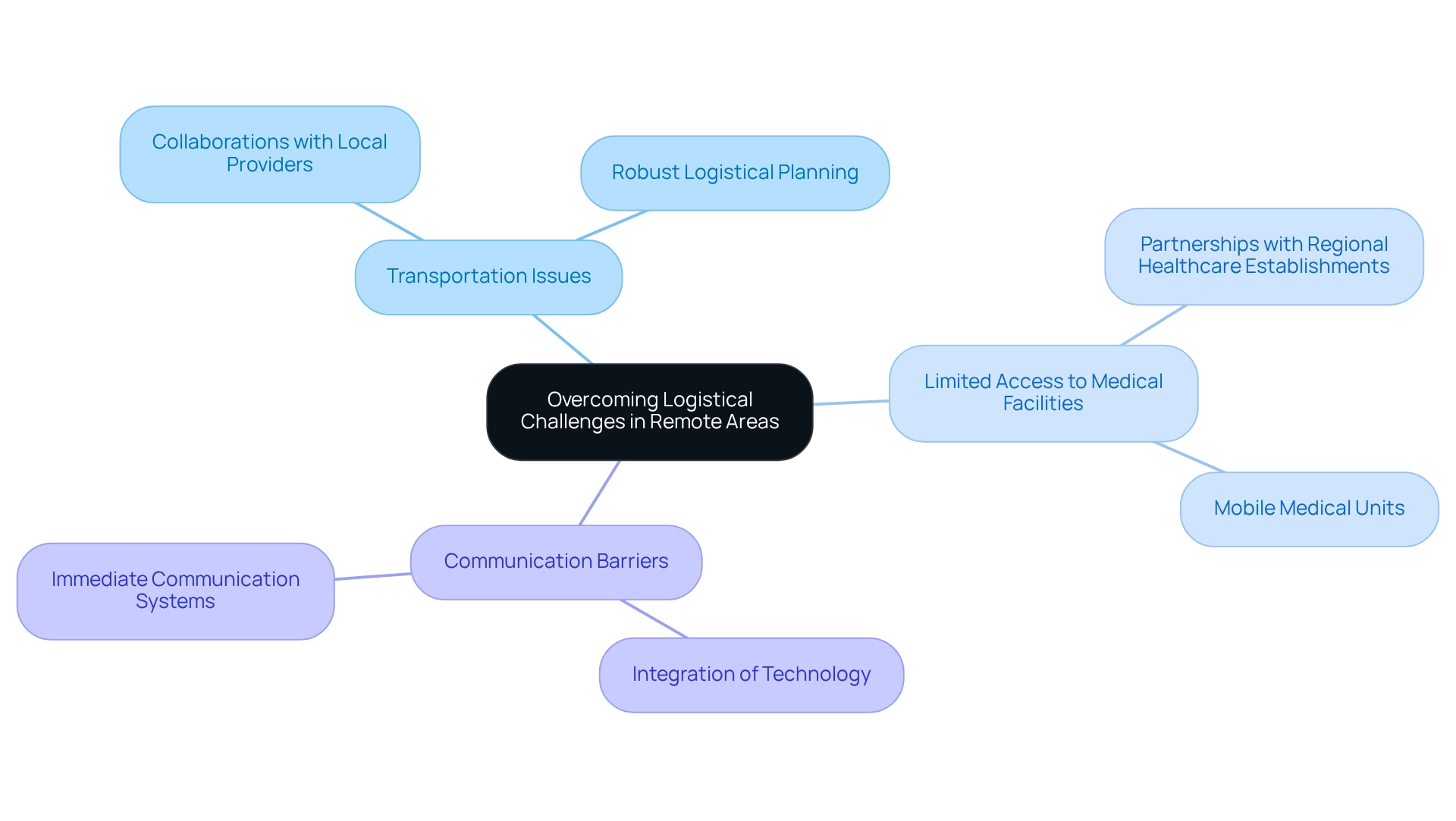
There are significant logistical challenges in designing trials for Bolivia, especially when conducting clinical studies in remote areas. The challenges in designing trials for Bolivia include:
- Transportation issues
- Limited access to medical facilities
- Communication barriers
To effectively navigate these obstacles, the company leverages over 20 years of expertise in robust logistical planning. This strategic approach includes forming collaborations with local transportation providers and regional healthcare establishments, ensuring effective setup and adherence to protocols. Moreover, the integration of technology facilitates immediate communication and monitoring, which significantly reduces delays and guarantees efficient adherence to study protocols.
With a focus on Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess® is exceptionally positioned to enable successful research in these challenging environments.
Implementing Effective Communication Strategies
Implementing effective communication strategies is essential for overcoming the challenges in designing trials for Bolivia. This necessity includes developing materials that are culturally appropriate and easily understandable for participants. Bioaccess® underscores the importance of training staff in communication techniques that foster trust and clarity. Regular updates and feedback mechanisms significantly enhance participant engagement and retention throughout the trial process.
Furthermore, employing regional languages and dialects in communication materials guarantees inclusivity and comprehension. Understanding the challenges in designing trials for Bolivia, as well as the regional healthcare landscape and regulatory environment, is also crucial. As highlighted by industry experts and media attention from Clinical Leader, a proactive strategy that integrates local perspectives can greatly enhance the efficiency of medical studies.
Ultimately, this approach contributes to job creation, economic growth, and healthcare improvement in the region.
Ensuring Data Integrity and Quality in Trials
Ensuring data integrity and quality in clinical studies is paramount. Bioaccess® employs stringent data management methods, incorporating frequent audits and validation checks to uphold the precision and dependability of study data across various types, including:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
Training local staff on data collection protocols and the importance of compliance with Good Clinical Practice (GCP) guidelines is essential to our approach. Furthermore, the utilization of electronic data capture systems simplifies data gathering, enhances data quality by minimizing human error, and enables real-time monitoring. This ensures our research studies meet the highest standards of regulatory excellence.
Managing Timelines Effectively in Clinical Trials
Effectively managing timelines in clinical studies is paramount, necessitating meticulous planning and proactive oversight. Bioaccess® employs a comprehensive methodology that encompasses:
- Feasibility assessments
- Investigator selection
- Study setup
- Regulatory compliance
All aimed at streamlining execution. By leveraging advanced project management tools, we meticulously track progress and identify potential delays at the earliest stages. Our robust project management and monitoring practices ensure that every detail, from start-up approvals to the reporting of serious and non-serious adverse events, is handled with precision.
Regular communication with all stakeholders, including sponsors and regulatory bodies, fosters alignment on timelines and expectations. Additionally, executing contingency plans for potential setbacks not only sustains momentum but also ensures that studies remain on course, effectively addressing the challenges in designing trials for Bolivia that are commonly faced by medical device startups.
Training Local Staff for Compliance and Quality
One of the challenges in designing trials for Bolivia is educating regional personnel, which is a vital component of successful clinical studies. Bioaccess® is committed to extensive training programs that prepare participants for the challenges in designing trials for Bolivia, focusing on:
- Regulatory compliance
- Data management
- Ethical considerations
These elements are crucial for navigating the complex landscape of medical device testing. By equipping local personnel with essential skills and knowledge, Bioaccess® ensures that studies are conducted to the highest standards, effectively addressing the challenges in designing trials for Bolivia and fostering compliance.
Specialized training in site feasibility and investigator selection further amplifies the effectiveness of these studies. Continuous training and support sustain quality throughout the testing process, ensuring efficient project management and reporting while cultivating a culture of excellence among research teams.
Adopting Adaptive Trial Designs for Flexibility
Adopting adaptive study designs is essential for enhancing flexibility in clinical research, allowing for modifications to protocols based on interim results. This approach not only leads to more effective tests but also improves participant outcomes. Bioaccess® strongly advocates for the implementation of adaptive designs, especially to overcome the challenges in designing trials for Bolivia.
By integrating real-time data analysis and feedback systems, researchers can make informed decisions that enhance the study's effectiveness and responsiveness to participant needs. Furthermore, partnerships like that of bioaccess™ and Caribbean Health Group play a pivotal role in establishing Barranquilla as a leading hub for medical studies in Latin America, supported by the Colombian Minister of Health.
This collaboration not only enriches the clinical trial landscape but also ensures the effective implementation of adaptive trial designs, leveraging bioaccess®'s extensive expertise in managing Early-Feasibility, First-In-Human, and other critical studies.
Conclusion
The strategies employed by bioaccess® underscore the significance of a tailored approach to clinical trials in Bolivia. By adeptly navigating the intricate regulatory landscape, effectively managing participant recruitment, and addressing logistical challenges, bioaccess® guarantees that trials are not only efficient but also culturally respectful and ethically sound. Their unwavering commitment to training local staff and implementing rigorous data integrity measures further enhances the quality of clinical research in the region.
The focus on adaptive trial designs exemplifies bioaccess®'s dedication to flexibility and responsiveness—crucial factors in achieving successful outcomes in clinical research. Collaborations with local healthcare providers and innovative communication strategies significantly enhance participant engagement and trust, both vital for trial success.
In conclusion, bioaccess® emerges as a leader in the Latin American clinical trial arena, skillfully supporting Medtech innovations while fostering local engagement and compliance. Their comprehensive approach not only accelerates the market entry of medical devices but also contributes to the overall enhancement of healthcare in Bolivia, paving the way for future advancements in medical research and patient care.
Frequently Asked Questions
What services does the company provide for clinical trials in Bolivia?
The company offers a comprehensive range of services, including feasibility studies, selection of research locations and principal investigators, regulatory compliance, study set-up, and project management.
How does the company ensure compliance with local regulations in Bolivia?
The company has a deep understanding of local regulations and cultural nuances, which allows them to streamline processes. They oversee approvals from ethics committees and health ministries, manage import permits, and ensure the nationalization of investigational devices.
What is the role of the National Medicines Agency (ANM) in clinical research in Bolivia?
The ANM is a regional regulatory authority that provides guidelines essential for study sponsors. Understanding their role and engaging with local experts can facilitate smoother approvals and compliance for clinical trials.
How does the company address data protection and client inquiries?
Clients can contact the Grievance Officer for any inquiries related to data protection, ensuring transparency and adherence to applicable laws.
What strategies does the company use to recruit qualified participants in Bolivia?
The company employs a multifaceted approach that includes leveraging regional healthcare networks, engaging in community outreach, and using culturally sensitive communication to foster trust and enhance participant involvement.
What partnerships does the company have to improve recruitment efforts?
The company collaborates with Caribbean Health Group, which has helped achieve over a 50% reduction in recruitment time and 95% retention rates, positioning Barranquilla as a leading site for research studies in Latin America.
What types of research studies does the company specialize in managing?
The company manages various types of research, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies.




