Introduction
Navigating the regulatory pathways for medical device approval can be daunting, especially when determining the appropriate submission process. The 510(k) and De Novo submissions represent two critical avenues for manufacturers seeking FDA clearance, each with distinct requirements and implications. A 510(k) submission is designed for devices that can demonstrate substantial equivalence to a legally marketed predicate, involving a streamlined process that typically takes 3 to 6 months.
On the other hand, the De Novo pathway caters to novel devices without a predicate, necessitating a thorough risk-based classification and often requiring a longer review period. This article delves into the intricacies of both submission types, comparing their timeframes, costs, regulatory burdens, and the critical role of risk management. By understanding these pathways, manufacturers can better navigate the FDA's regulatory landscape, ensuring compliance and expediting the approval process for innovative medical devices.
What is a 510(k) Submission?
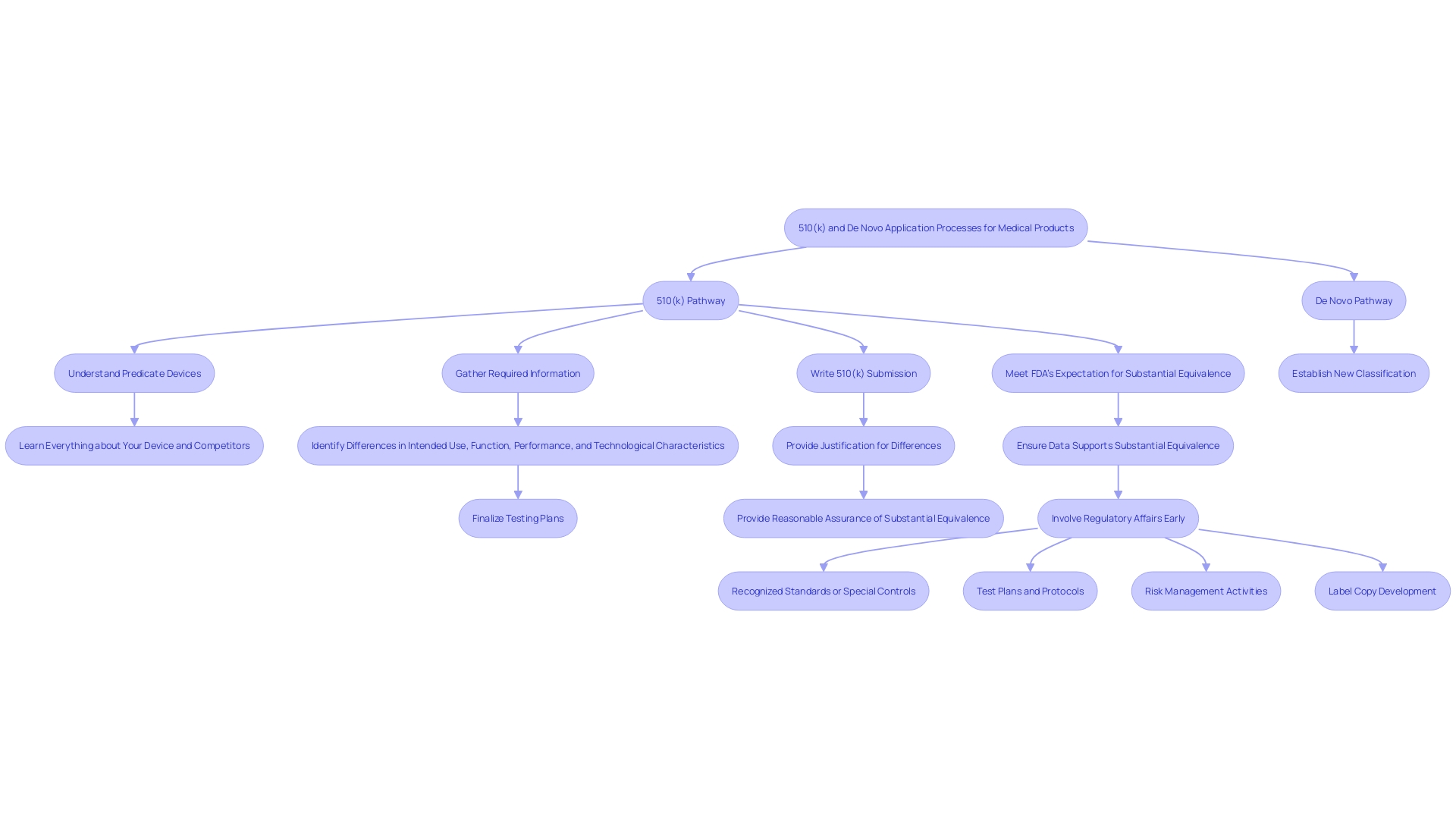
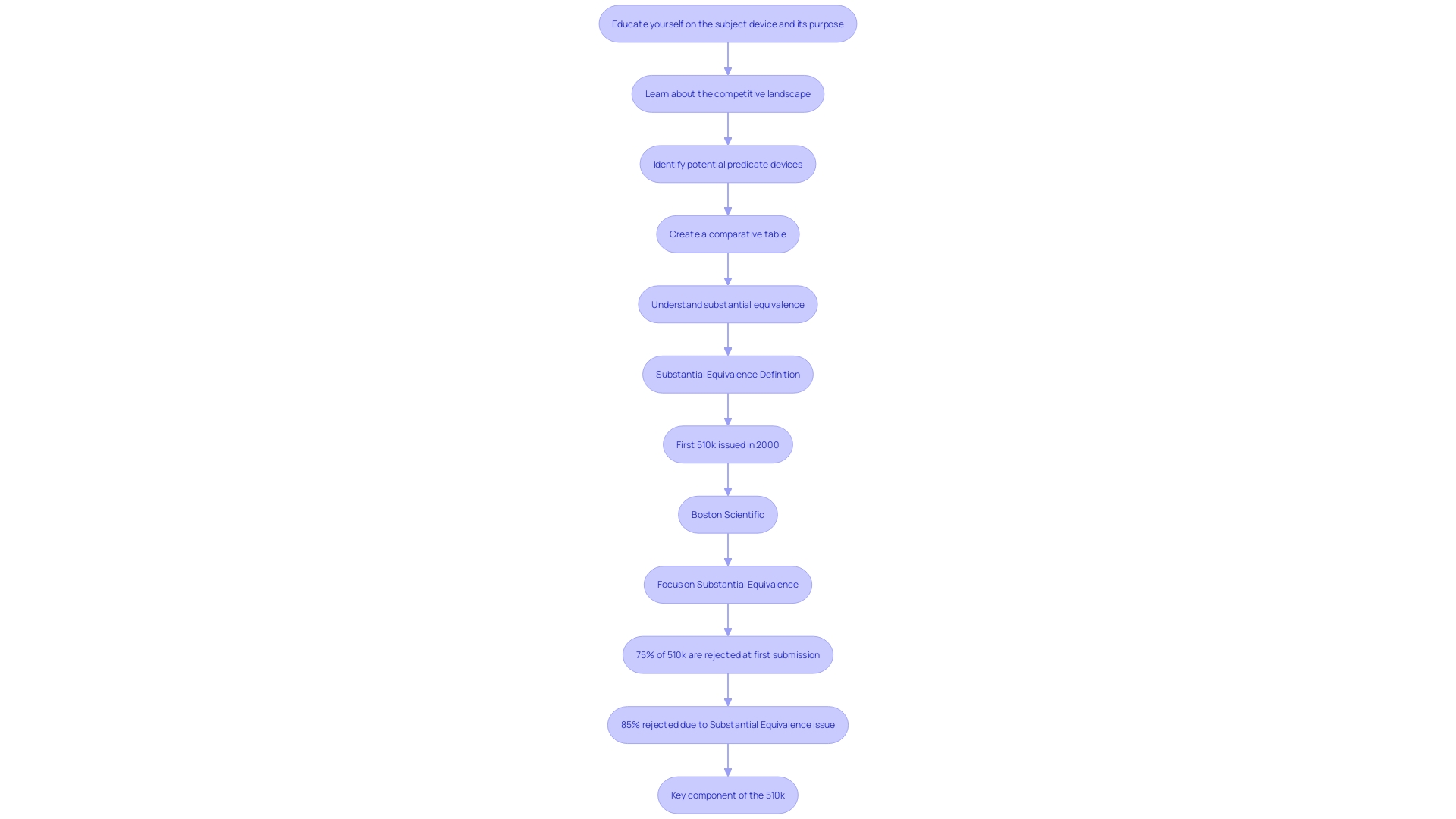
A 510(k) submission is a premarket notification made to the FDA to demonstrate that a medical instrument is safe, effective, and substantially equivalent to a legally marketed item. To achieve this, manufacturers must provide comprehensive evidence that their product performs as intended. This includes data from various testing methodologies such as bench tests, animal studies, or clinical trials. The process requires a thorough understanding of the item's intended use, warnings, and cautions, as well as the competitive landscape. Manufacturers must identify potential predicate devices with similar functionalities and create a comparative table to highlight similarities and differences. Typically, this streamlined pathway takes around 3 to 6 months for approval, contingent on the completeness of the application. The FDA's expectations and guidance documents are essential for applicants to follow to ensure their proposal meets the agency's standards, thereby facilitating a decision of substantial equivalence.
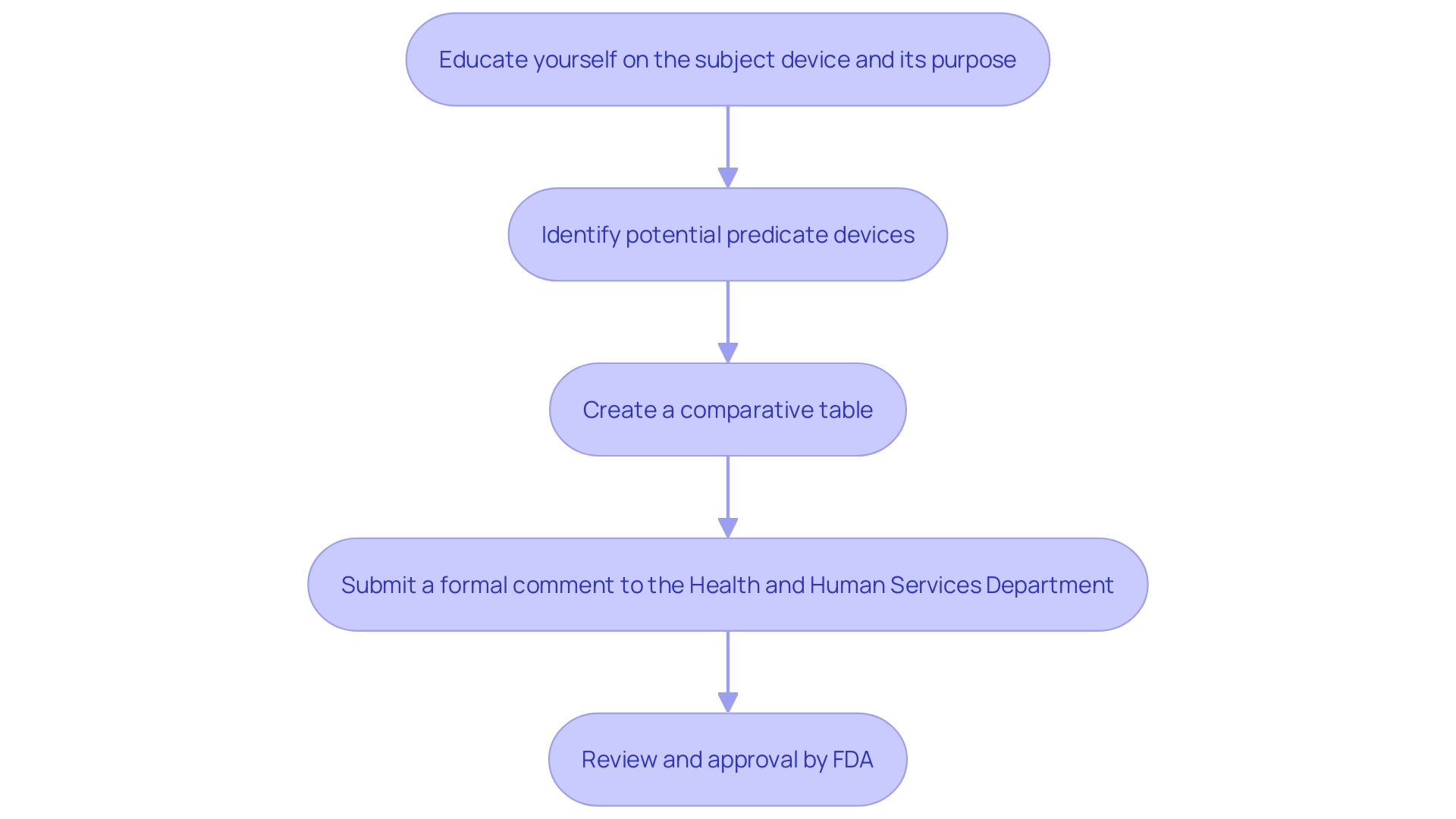
What is a De Novo Submission?
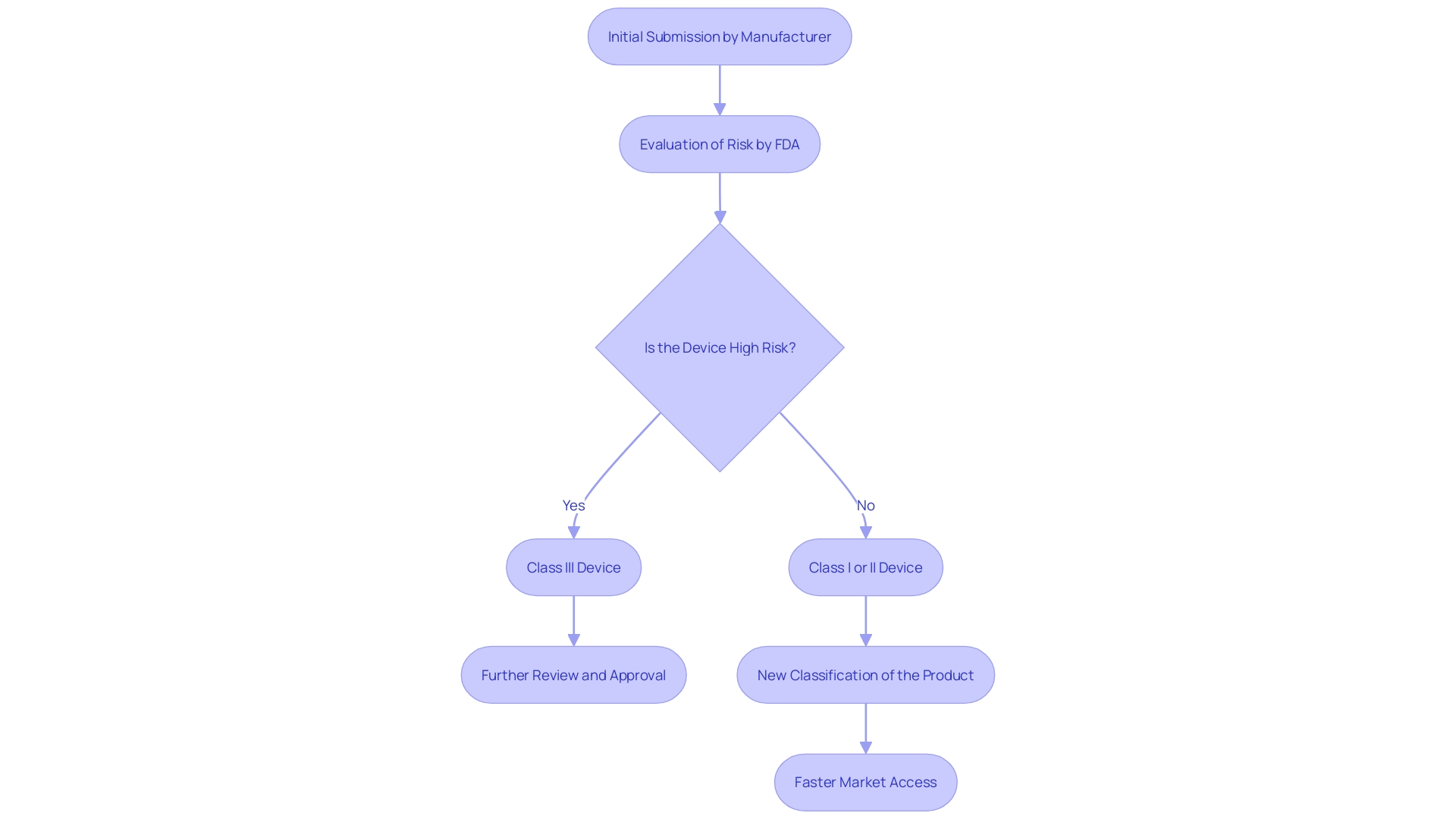
The De Novo submission process is a critical pathway for novel medical tools that lack a predicate to claim substantial equivalence. This process enables manufacturers to seek a risk-based classification for their product, which can result in a new classification if the FDA concludes that the product presents a low to moderate risk. This tailored regulatory approach is particularly beneficial for unique items that do not fit into existing categories. Based on recent reports, the FDA has released fifteen new decision summaries for new applications, emphasizing the agency's continuous efforts to simplify the approval process and encourage innovation in the healthcare equipment sector. This process not only permits a more tailored regulatory pathway but also guarantees that new technologies can access the market more rapidly, thereby speeding up progress in healthcare.
Key Differences Between 510(k) and De Novo Submissions
'The difference between 510(k) and De Novo applications is essential for comprehending the regulatory routes for medical products.'. A 510(k) application depends on showing that a new instrument is substantially comparable to a predicate instrument already available. This means the new apparatus must have the same intended use and similar technological characteristics as the predicate apparatus or provide sufficient scientific data to prove it is as safe and effective.
In contrast, new applications are utilized for items that lack a predicate. This process involves establishing a new classification for the equipment and a more comprehensive evaluation of its risk profile. Due to the need for this extensive review, De Novo applications generally take longer to process than 510(k) applications.
Significantly, the 510(k) pathway is simplified as it depends on current classifications and safety information from predicate products. However, this can be challenging, as approximately 75% of 510(k) applications are initially rejected, with 85% of these rejections stemming from issues related to substantial equivalence. This highlights the importance of thoroughly understanding the competitive landscape and the technological characteristics of potential predicate devices to avoid such pitfalls.
De Novo vs 510(k): Time and Cost Considerations
The duration and expenses related to 510(k) applications are typically lower than those of new device processes. On average, a 510(k) review takes about 3 to 6 months. In contrast, new reviews can take anywhere from 6 months to over a year. Regarding costs, 510(k) applications often necessitate less initial investment in clinical data. In contrast, new applications may require extensive clinical trials and data gathering, leading to increased expenses.
Comparing Regulatory Burden and Complexity
The regulatory burden associated with 510(k) submissions is notably less complex compared to De Novo submissions. The 510(k) pathway is well-established with clear guidelines for demonstrating substantial equivalence to a predicate product. According to the FDA, substantial equivalence means that the new device has the same intended use and technological characteristics as a legally marketed device, or that any differences do not raise new questions of safety and effectiveness. Regardless of this, statistics indicate that 75% of 510(k) applications are initially denied, with 85% of these denials resulting from problems associated with substantial equivalence during scientific evaluation.
In contrast, new submissions involve a more comprehensive analysis of risk and benefit, requiring detailed justifications for the proposed classification. This process can be more daunting for manufacturers, particularly those less familiar with regulatory requirements. The De Novo pathway is utilized for new products that lack an appropriate predicate, requiring a more detailed risk evaluation and frequently more comprehensive clinical data.
Adjusting to these regulatory environments requires a profound comprehension of the equipment, its users, and the competitive landscape. Industry experts suggest making comparative tables and carefully examining the Summaries of Safety and Effectiveness (SSEs) found in the FDA’s 510(k) database to assess similarities and differences with predicate products. This methodical approach not only streamlines the documentation process but also ensures compliance and minimizes delays.
Understanding the De Novo Classification Process
The new classification procedure provides a route for innovative medical instruments that do not have a reference item. When a manufacturer submits a De Novo request to the FDA, the dossier must include comprehensive data about the product's safety and efficacy. The FDA then evaluates whether the apparatus poses low to moderate risk and determines the appropriate regulatory controls needed to ensure its safety and effectiveness. If the FDA approves the request, the product is categorized and can act as a predicate for future 510(k) submissions. This pathway is especially important considering that more than 1.7 million injuries and 83,000 fatalities in the United States over a decade were possibly associated with healthcare instruments, emphasizing the need for comprehensive pre-market evaluations and regulatory measures. The FDA's role in this process emphasizes its wider mission to safeguard public health by ensuring the safety and effectiveness of health-related products.
Who Qualifies for FDA's De Novo Pathway?
'The new pathway provides an efficient way for producers looking to launch innovative medical products that do not have a current counterpart.'. This pathway is particularly suitable for devices classified as low to moderate risk, ensuring they are both safe and effective for their intended use. To qualify, manufacturers must present comprehensive scientific evidence, including data from preclinical or clinical studies, to substantiate their request for classification.
Recent updates highlight the dynamic nature of the new process. For instance, as of August 28, fifteen new decision summaries have been posted, with more than half originating in 2023. This illustrates both the FDA's continuous dedication to openness and the growing use of the new pathway by producers. 'The FDA's role in safeguarding public health extends to ensuring the efficacy and safety of these health products, reinforcing its mission to protect and promote public health through rigorous regulatory oversight.'.
'The significance of the De Novo classification is further highlighted by historical information, which suggest that over a ten-year span, more than 1.7 million injuries and 83,000 fatalities in the United States were potentially associated with healthcare instruments.'. This statistic emphasizes the critical need for thorough evaluation and regulation of new healthcare technologies. 'The FDA's efforts in this regard are crucial in mitigating risks associated with medical equipment and enhancing patient safety.'.
Risk Management and Benefit-Risk Analysis in De Novo Submissions
Risk management and benefit-risk analysis are pivotal components of the De Novo submission process. Manufacturers must meticulously evaluate potential risks associated with their product and demonstrate that the benefits significantly outweigh these risks. This entails a thorough evaluation of the apparatus's performance, possible negative impacts, and its ability to meet unfulfilled healthcare requirements. The FDA places substantial emphasis on these analyses to ensure that only items with a favorable benefit-risk profile receive approval. As Bijan Elahi, a specialist in safety risk management for healthcare tools, observes, 'Establishing risk acceptance standards and deciding when to cease risk mitigation are essential components in guaranteeing equipment safety.' Furthermore, the FDA's strict regulatory structure seeks to safeguard public health by guaranteeing the safety, effectiveness, and security of health products, a duty emphasized by the agency's dedication to thorough supervision. Significantly, over a decade, more than 1.7 million injuries and 83,000 fatalities in the United States were possibly associated with healthcare tools, emphasizing the essential requirement for comprehensive risk management. Thus, the emphasis on detailed and coherent presentation of clinical data and post-market surveillance is indispensable in aligning with FDA requirements and ensuring the safety of medical devices.
Conclusion
Navigating the regulatory pathways for medical device approval requires a comprehensive understanding of both the 510(k) and De Novo submission processes. The 510(k) submission is primarily aimed at demonstrating substantial equivalence to a legally marketed device, allowing for a streamlined approval process that typically takes between 3 to 6 months. In contrast, the De Novo pathway is designed for novel devices that lack a predicate, necessitating a thorough risk-based classification and often involving a more extensive review period.
The key differences between these two submission types highlight the regulatory challenges that manufacturers face. While the 510(k) process is generally less complex and less costly, it is not without its hurdles, as evidenced by the high rate of initial rejections. The De Novo pathway, while more tailored for innovative devices, involves a comprehensive analysis of risk and benefit, requiring detailed justifications and extensive clinical data.
This complexity underscores the importance of meticulous preparation and understanding of both the device and its competitive landscape.
Ultimately, the choice between a 510(k) and De Novo submission should be informed by the device's characteristics and intended use. Manufacturers must weigh the time, cost, and regulatory burden associated with each pathway to ensure compliance and expedite market entry. By leveraging a thorough understanding of these submission processes, manufacturers can better navigate the FDA's regulatory landscape, fostering innovation while prioritizing public health and safety.




