Introduction
21 CFR Part 812 is a crucial regulatory framework that governs clinical investigations involving investigational devices. It outlines the responsibilities of sponsors, investigators, and institutional review boards (IRBs) to ensure the safety and efficacy of the devices under study. This article provides an in-depth overview of the key components of 21 CFR Part 812, including the purpose and scope of the regulation, the IDE application and approval process, the differences between significant risk and nonsignificant risk devices, exemptions from IDE requirements, labeling and distribution requirements, informed consent and subject protection, monitoring and reporting responsibilities, quality management systems (QMS) and compliance, as well as common challenges and best practices for IDE compliance.
By understanding and adhering to these regulations, sponsors and investigators can conduct clinical investigations that prioritize patient safety and contribute to the advancement of medical science.
What is 21 CFR Part 812?
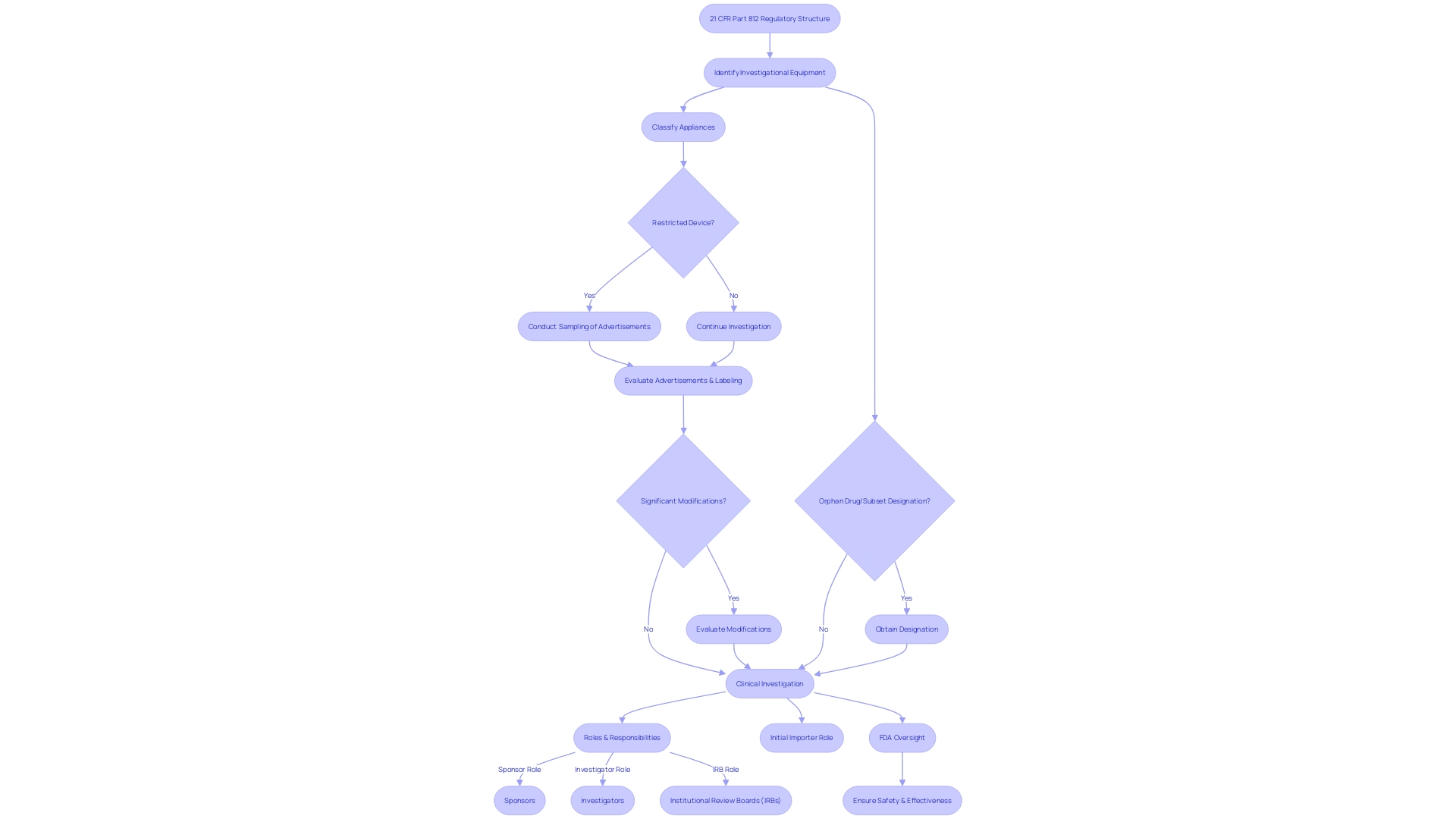
21 CFR Part 812 covers the regulatory structure necessary for the supervision of clinical investigations involving investigational equipment. It outlines the duties and necessary processes for sponsors, investigators, and institutional review boards (IRBs) to ensure the safety and effectiveness of the equipment being studied. The section specifies thorough explanations vital to the regulatory process, such as 'restricted object,' which pertains to any item with imposed limitations on sale, distribution, or use as mandated by specific regulations set forth under the Federal Food, Drug, and Cosmetic Act. Moreover, it establishes 'classification name,' which the FDA employs to classify appliances, and 'product code,' a distinctive identifier for appliance categorization. It also outlines what constitutes 'representative sampling' of advertisements and labeling, providing guidance on the promotional material that conveys the product's claims. Impoftantly, the regulation covers 'significant modifications' that may impact the identity of the effectiveness and profile of a product, such as changes to the label, components, intended purpose, contraindications, or usage guidelines.
The initial importer plays a crucial role in the supply chain, being the first point of contact within the U.S. for an item entering from abroad, without altering the item's original packaging or labeling. The FDA ensures that the public health is protected by confirming the safety, effectiveness, and security of medical equipment. This is embodied in their rigorous oversight of the nation's food supply, pharmaceuticals, and other health-related products. To safeguard the integrity of treatments for rare conditions, 'orphan drug' and 'orphan subset' designations are used to identify drugs meant for rare diseases or subsets within non-rare conditions, respectively. These designations are crucial as they come with exclusive approval rights that encourage the development of therapies for rare diseases by ensuring market exclusivity for a period post-approval. The standards for determining whether a subsequent product is the same as a previously approved one is rigorous, taking into account factors such as molecular structure and superiority.
Purpose and Scope of 21 CFR Part 812
'21 CFR Part 812 is crucial to the regulation of investigational devices, outlining the responsibilities and guidelines for sponsors, investigators, and Institutional Review Boards (IRBs). Within its framework, sponsors are required to submit an Investigational Device Exemption (IDE) application, a pivotal step before commencing clinical trials. This regulation meticulously outlines the IDE process, including the need for representative samples of advertisements and any other labeling to reflect the promotional claims made for the product accurately. Furthermore, any significant alterations in the labeling or advertising of the product, which may affect its identity or safety and efficacy, must be reported.
The regulation also emphasizes the importance of the classification name, which describes a device or class of devices, and the product code, which the FDA uses to identify a device's generic category. These elements are essential for preserving the integrity and compliance of investigations in the medical field. The scope of Part 812 spans the entire clinical investigation continuum, from the initial IDE application to the final reporting of study results, encompassing all stages to ensure the protection and well-being of human subjects.
The FDA's purpose is to ensure the well-being, effectiveness, and security of medical equipment, among other responsibilities. As such, the agency has recently updated its standards for direct-to-consumer prescription drug advertisements to ensure clear, conspicuous, and neutral presentation of major side effects and contraindications in TV and radio formats, echoing the principles of transparency and patient well-being that underpin 21 CFR Part 812.
It is important to mention that the FDA's supervision of medical product safety, which includes medical equipment, is a high-priority matter. Research has brought attention to the possible connection between medical equipment and a substantial amount of harm and fatalities, emphasizing the crucial requirement for strict regulatory frameworks such as 21 CFR Part 812. The regulation serves as a foundation to safeguard subjects in investigations, ensuring that the potential benefits to subjects and the value of the knowledge to be gained justify the risks involved.
Key Components of 21 CFR Part 812
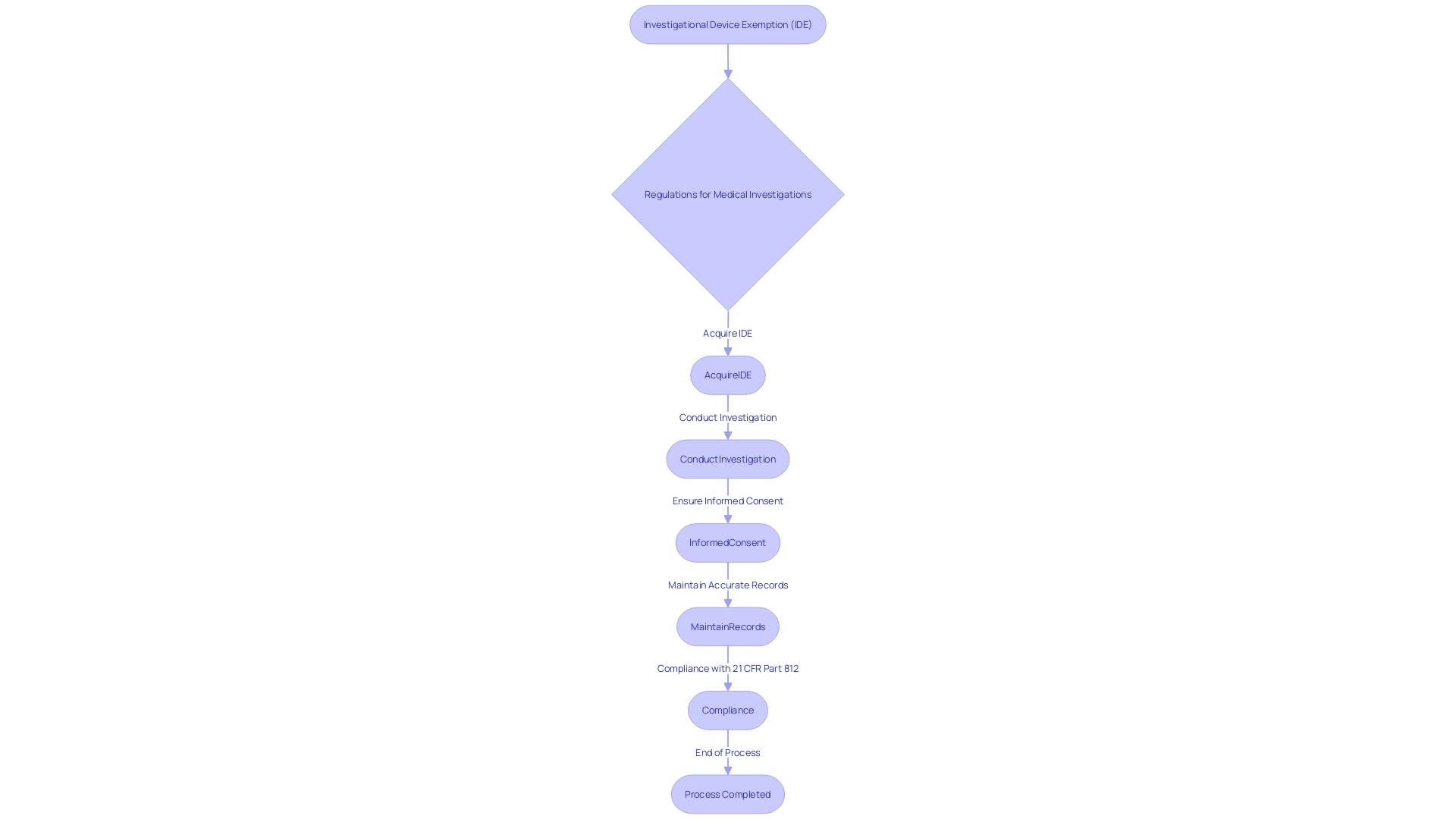
The complexities of 21 CFR Part 812 are crucial for research professionals in the field of investigation, covering a range of components that establish the basis of studies on experimental tools. Commencing with the description of a research instrument, these regulations outline the requirements for acquiring an Investigational Instrument Exemption (IDE), which allows a medical instrument to be employed in a study to gather data on well-being and efficacy. The procedures for conducting the investigation are methodically outlined, ensuring that the study design adheres to the highest scientific standards.
Moreover, the regulation delineates the respective responsibilities of sponsors, investigators, and Institutional Review Boards (IRBs), creating a framework for accountability and oversight. The informed consent process is particularly emphasized, requiring clear communication to participants about the risks and benefits of the study. Additionally, the maintenance of meticulous records and the submission of accurate reports are mandated to uphold the integrity of the trial and to ensure compliance with FDA regulations.
Recent FDA updates further underscore the importance of presenting drug information in a clear, conspicuous, and neutral manner, as seen in the "Direct-to-Consumer Prescription Drug Advertisements" rule. This reflects an overarching commitment to transparency and patient well-being, which resonates throughout 21 CFR Part 812. The FDA's responsibility in protecting public health by guaranteeing the safety and efficiency of medical equipment is crucial to these regulatory elements, which as a whole direct the ethical and scientific behavior of trials.
IDE Application and Approval Process
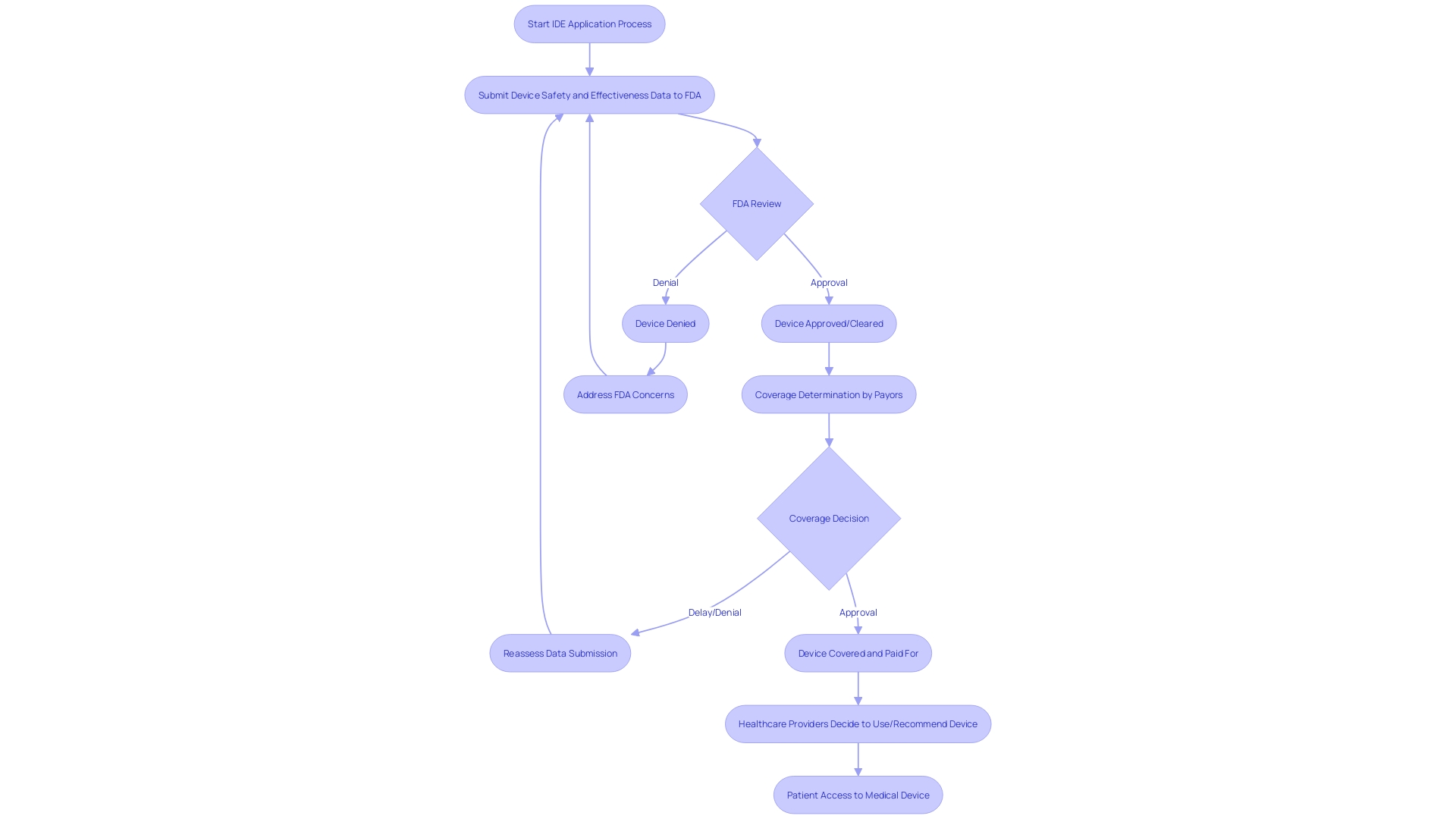
Navigating the complexities of an Investigational Device Exemption (IDE) application under 21 CFR Part 812 is crucial for trial sponsors and investigators. An IDE application is a comprehensive file that includes details ranging from the description of the apparatus, manufacturing information, to the proposed study protocol. The FDA's role goes beyond evaluating the safety and efficacy of medical devices; it also involves assessing the application's adherence to robust software engineering practices crucial for the reliability and scalability of biomedical tools in the context of trials.
For example, an IDE application for an investigation that seeks to alter health policies or standards of care must present comprehensive evidence. This includes clinical trial applications with annual direct costs exceeding $700,000, underlining the financial magnitude and implications of such research. The FDA review process is stringent, with the agency granting orphan-drug designation under section 526 of the act, ensuring seven years of market exclusivity, barring any clinically superior subsequent drugs.
In the realm of medical equipment, coverage and payment decisions by payors after FDA approval can be influenced by the data manufacturers submit to demonstrate a product's safety and effectiveness. Given that this information may not meet the criteria of payors, it could result in delays or denials in the availability of the product.
Chris, a biomedical engineer with vast experience in overseeing studies for Class III equipment, emphasizes the significance of adjusting and updating software tools to harness new technologies and computing models - a hurdle often encountered due to resource constraints. This echoes the FDA's initiative in emphasizing the need for reliable and efficient software tools, a sentiment that is increasingly shared among stakeholders in the field.
To this end, the eCFR's published edition presents information in an indented format for user convenience, ensuring clarity in the interpretation of regulatory documents. These insights are crucial for sponsors and investigators to understand the IDE application process, positioning them to effectively initiate and conduct their investigations.
Differences Between Significant Risk and Nonsignificant Risk Devices
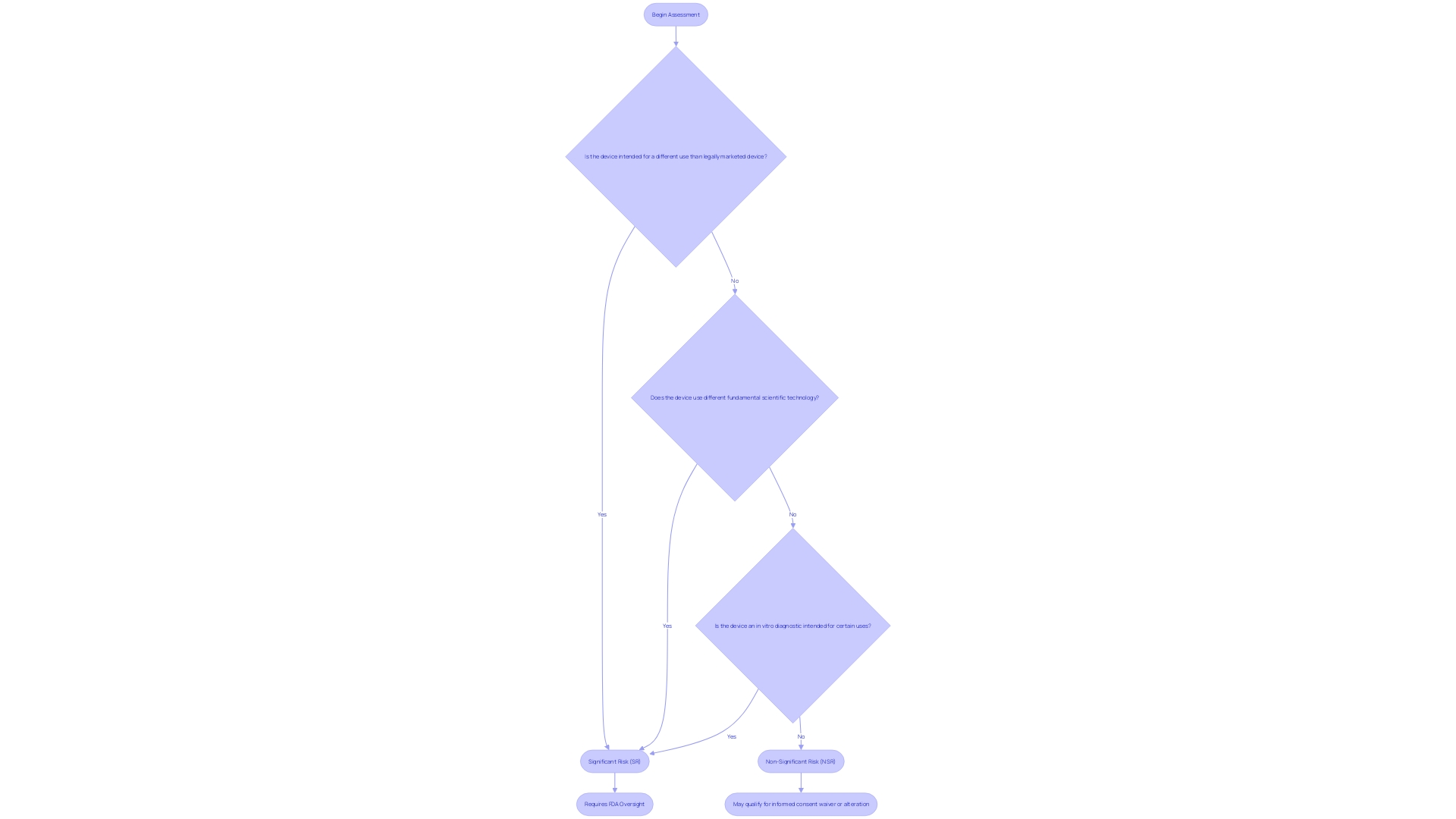
Under the jurisdiction of 21 CFR Part 812, investigational medical instruments are thoroughly categorized based on the level of risk they pose to participants in a clinical study. Devices are categorized as either significant risk (SR) or non significant risk (NSR), and this distinction is pivotal in determining the extent of regulatory oversight required. For objects considered to be of notable risk, strict supervisory measures are in place to safeguard participant well-being, while those evaluated as insignificant risk are subject to less rigorous regulatory controls. The criteria for this risk classification encompass a range of factors, including the intended use of the device, its technological complexity, and the potential for harm. Sponsors and investigators must be skilled at distinguishing these classifications to ensure their research adheres to the appropriate regulatory standards and upholds the highest safety protocols for trial participants.
Exemptions from IDE Requirements
To ensure the swift and compliant progression of clinical investigations, it is imperative to recognize instances where an Investigational Device Exemption (IDE) may not be a requisite under 21 CFR Part 812. For instance, the Impella Connect System, which includes a web-based portal and hardware for remote monitoring in critical care, is classified as an item necessitating premarket authorization due to its life-critical alarm functions. Likewise, exceptions are given to authorized professionals who produce or alter instruments exclusively for their profession, retail stores such as pharmacies that provide instruments directly to customers, and organizations engaged in producing instruments solely for research, education, or analysis with no commercial distribution. Moreover, carriers engaged in the regular conduct of business and service providers who distribute products to the end user are exempt. These exemptions are essential for sponsors and investigators to navigate, as they provide clarity on when an IDE is not mandatory, thereby streamlining the initiation of clinical trials without compromising on regulatory adherence.
Labeling and Distribution Requirements
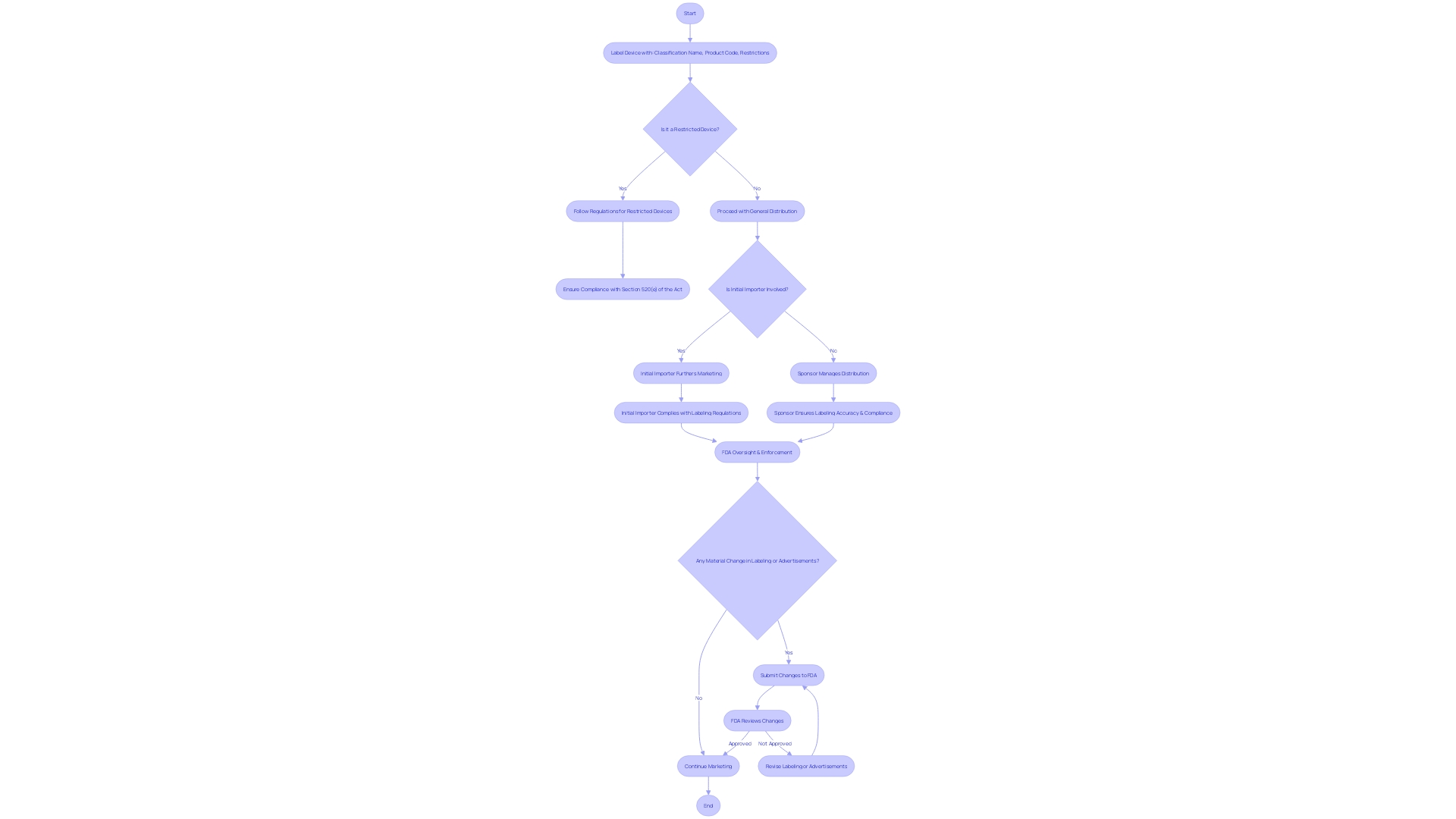
Labeling and distribution protocols for investigational instruments, as mandated by 21 CFR Part 812, are fundamental to maintaining compliance with FDA regulations. Labels must clearly convey essential information, such as the classification name, product code, and any restrictions on sale, distribution, or use as per FDA guidelines. This guarantees that investigational instruments are accurately identified and that their safety and effectiveness are not compromised by improper labeling.
Essential information, including promotional claims made for the product, must be represented in a 'representative sampling of advertisements' and any other labeling materials. These should exclude labels and package inserts but provide accurate depictions of the claims. Additionally, any significant alteration in labeling or advertisements that may impact the product's identity or performance must be recorded and evaluated for conformity.
Sponsors and initial importers have specific responsibilities under these regulations. Initial importers, who introduce an item from a foreign manufacturer to the U.S. market, must ensure they do not alter the packaging or labeling in a way that might affect compliance. Furthermore, they must report any event related to the manufacturing process of the equipment, such as deviations from good manufacturing practices or unforeseen circumstances that could affect the device's integrity, purity, or effectiveness.
Recent case studies illustrate the gravity of these requirements. For example, a firm's response to FDA's inquiry about complaint coding and quality data analysis was deemed inadequate due to the absence of outcomes from their corrective actions. This highlights the need for thorough documentation and evidence of compliance.
Furthermore, the FDA's responsibility in safeguarding public health by ensuring the safety and effectiveness of medical equipment serves as a reminder of the significance of strict compliance with labeling and distribution regulations. Every party involved in the process, from the sponsor to the investigator, must understand their responsibilities to ensure the proper handling and use of investigational devices throughout the investigation.
Informed Consent and Subject Protection
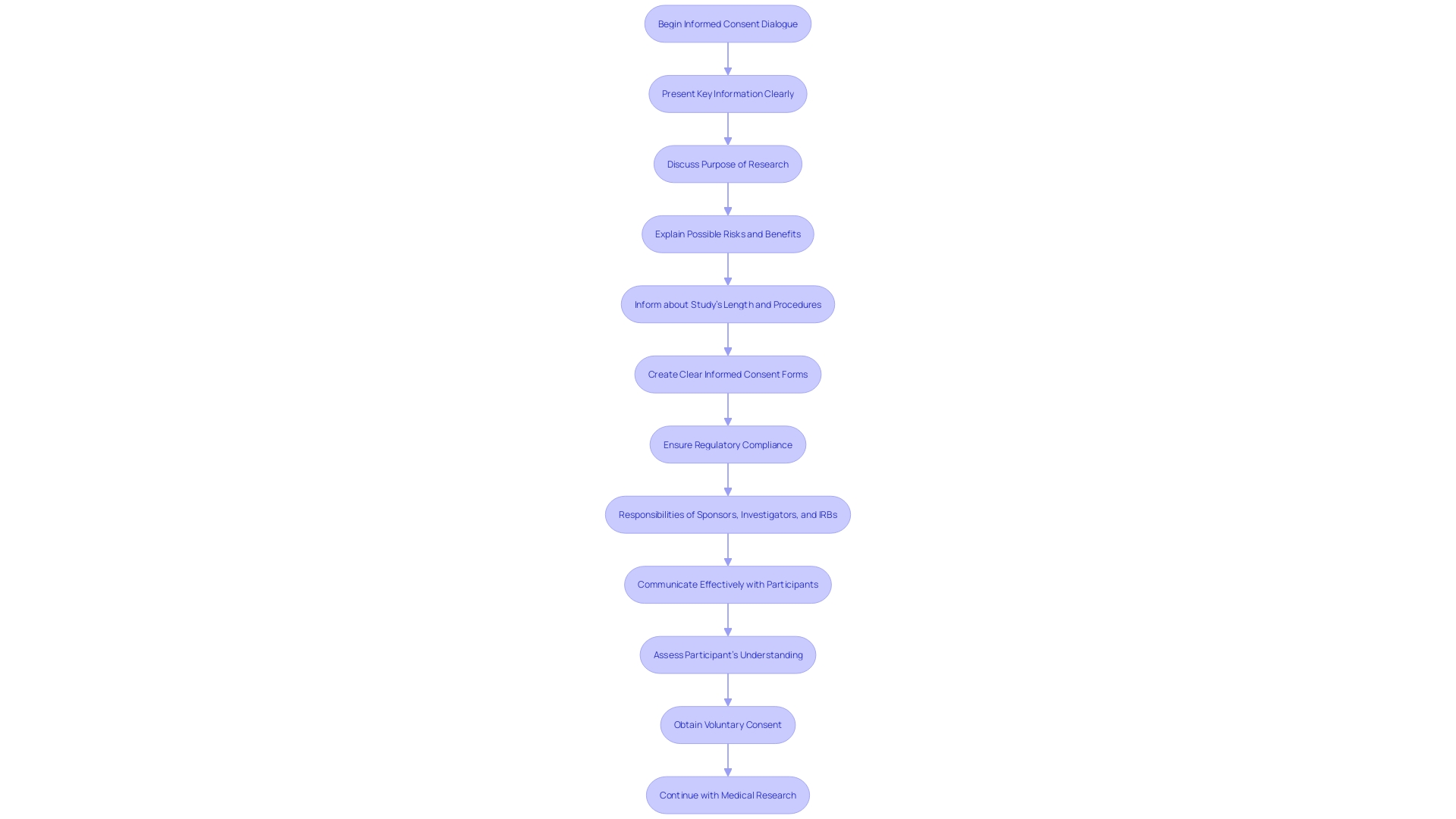
The ethical foundation of medical research is deeply rooted in the principle of informed consent, as delineated in 21 CFR Part 812. This crucial process involves a comprehensive dialogue between researchers and participants, ensuring individuals are fully apprised of the study's purpose, potential risks, benefits, and procedures. The informed consent form must encapsulate this information in a manner that is both clear and concise to support participants' understanding of their involvement. The safeguarding of human subjects is paramount, and regulatory compliance is integral to uphold this standard. The recent attention to informed consent in research has highlighted key aspects that need to be conveyed at the onset, such as study length and any pertinent information that aids participants in making an informed decision. This is in line with the fundamental tenets of the Declaration of Helsinki and the draft guidance issued by the FDA, which both underscore the significance of communicating the essence of the research in an accessible format. Moreover, the inclusion of key details in consent documents not only benefits potential participants but also serves as a valuable tool for those already involved in the study. The responsibility of conveying this information effectively falls upon sponsors, investigators, and IRBs, who must navigate these requirements with precision and care.
Monitoring and Reporting Responsibilities
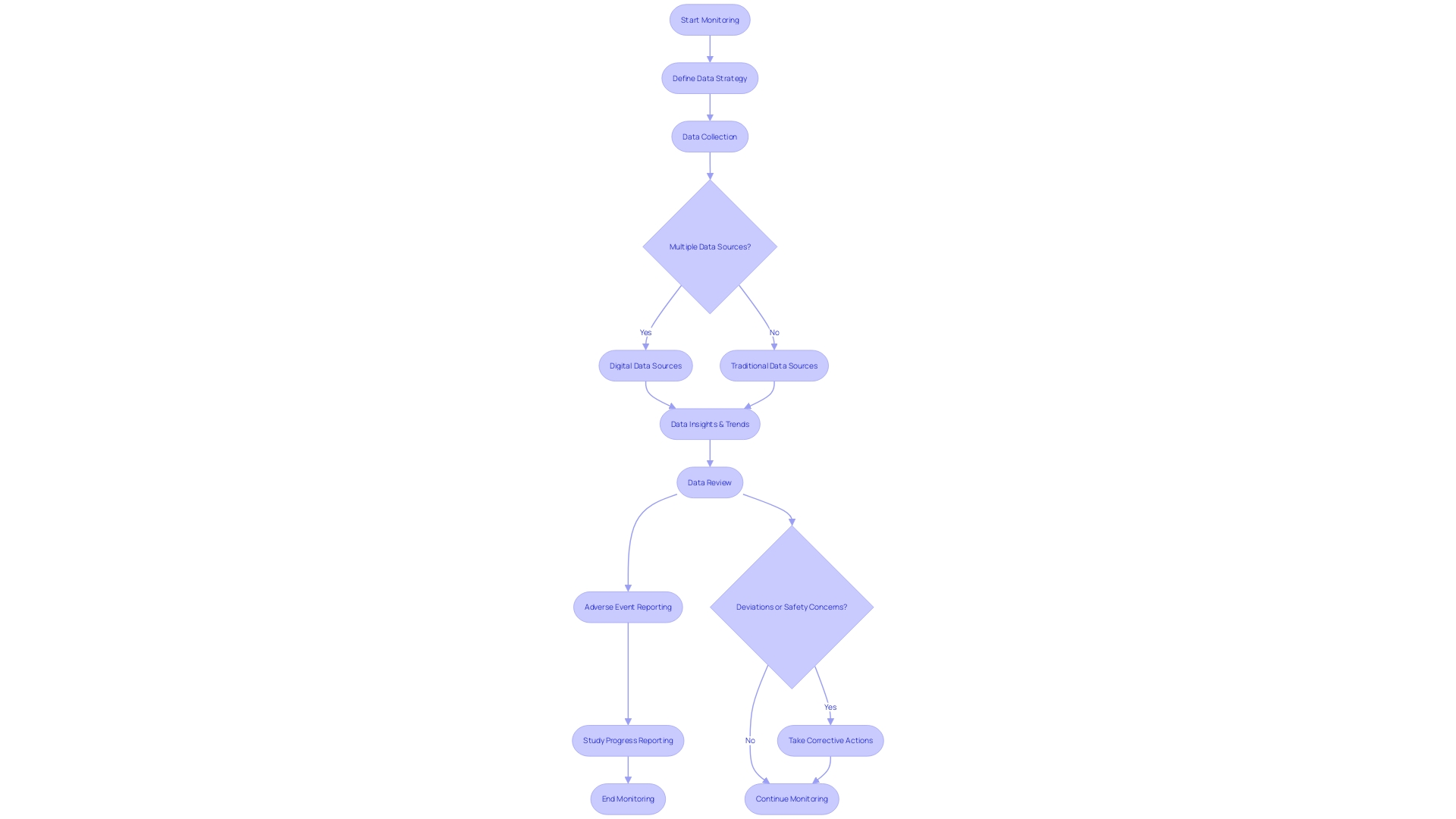
Following the Code of Federal Regulations (CFR) Title 21 Part 812 is crucial for the well-being and integrity of investigations. This section outlines the monitoring requirements for sponsors and investigators that are critical for maintaining compliance with these regulations. Responsibilities include meticulous data collection, prompt adverse event reporting, and regular study progress reporting. Precise monitoring ensures that any deviation from the current good manufacturing practice, applicable regulations, standards, or specifications that may impact the well-being, purity, or potency of a product is reported. This applies to events occurring not only within the sponsor's facility but also at any contracted facility.
The FDA holds a pivotal role in overseeing the conduct of trials to protect public health. For instance, the FDA recently established standards ensuring that direct-to-consumer prescription drug advertisements present major side effects and contraindications clearly and neutrally. This reflects the agency's commitment to transparency and security in all aspects of drug marketing, which aligns with the monitoring and reporting ethos in clinical trials.
In the event of a safety concern or regulatory violation, a comprehensive response plan, including public warnings and checks for effectiveness, must be implemented. The plan must detail the actions to be taken by the entity under a cease distribution and notification order or a mandatory recall order. This includes all necessary corrections, such as repair, modification, adjustment, relabeling, destruction, or inspection of the object without its physical removal from the point of use.
For health professionals engaged in clinical investigations, understanding the nuances of these regulations, such as the definitions of 'consignee,' 'correction,' 'device user facility,' and 'restricted device,' is of paramount importance. These terms outline who is responsible for the handling of medical equipment and the scope of their responsibilities. For example, 'initial importer' refers to any importer who furthers the marketing of a device to the final point of sale but does not alter the device's packaging or labeling.
The CFR also provides clarity on orphan-drug designation and exclusivity, ensuring that newly approved drugs for rare diseases are protected from competition for a specific period, fostering innovation and research in this critical area.
It is crucial for sponsors and investigators to stay informed about these regulatory requirements to ensure the continued safety of trials and adherence to high standards of practice.
Quality Management System (QMS) and Compliance
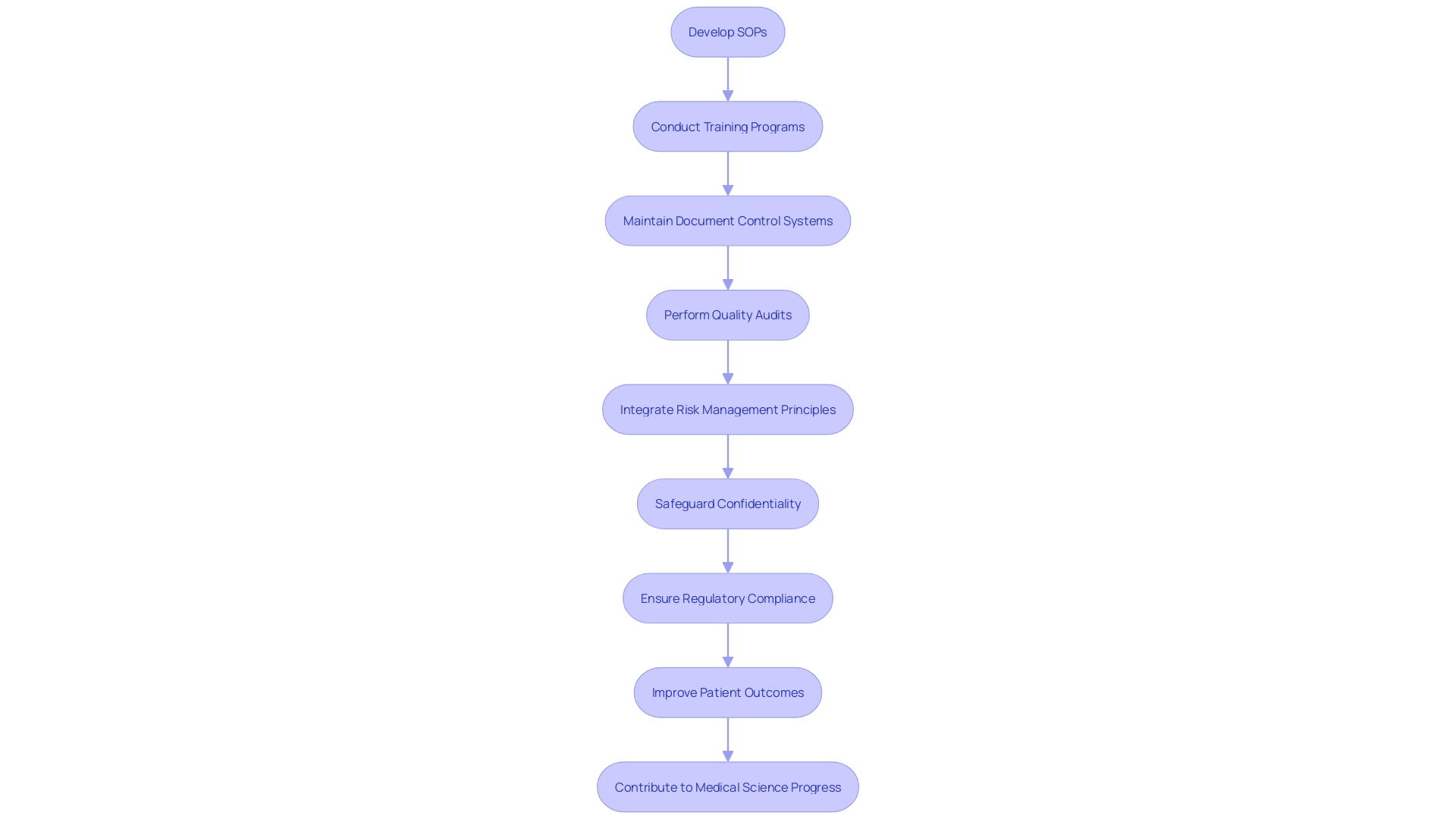
A robust Quality Management System (QMS) is pivotal in meeting the compliance requirements of 21 CFR Part 812. At the core of a QMS are the Standard Operating Procedures (SOPs), which provide a blueprint for conducting operations in a consistent and compliant manner. Essential to the integrity of the QMS, SOPs need to be well-documented and accessible, ensuring that all team members are informed and can perform their duties according to the established protocols.
Training programs are another cornerstone of a comprehensive QMS. They ensure that all personnel are equipped with the necessary knowledge and skills to maintain compliance with the changing landscape of trial regulations. By promoting a culture of continuous learning, organizations can better handle the risks associated with medical instrument development and clinical investigations.
Document control systems serve as the repository for all QMS documentation, ensuring that the most current procedures are used and that historical data is preserved for reference or audits. This careful method of recording information is not just a legal obligation but also a recommended approach that improves the effectiveness and efficiency of medical equipment.
Quality audits, both internal and external, are indispensable for verifying compliance with regulatory standards and identifying areas for improvement. Audits offer an opportunity for organizations to scrutinize their processes and make necessary adjustments to uphold the highest quality standards.
Furthermore, fostering a culture of compliance within the organization is not only about following regulations; it's about instilling a mindset where quality and patient well-being are paramount. Quality assurance plays a critical role in this context by ensuring that all aspects of the QMS align with regulatory expectations and contribute to the delivery of high-quality medical instruments.
The integration of risk management principles, as highlighted by Etienne Nichols, a Medical Device Guru, into the QMS fortifies the commitment to patient safety and product excellence. Adherence to standards such as ISO 14971 complements the QMS by providing a framework for risk assessment and mitigation throughout the device lifecycle.
In light of the public nature of comments and submissions to the FDA, as emphasized in recent FDA communications, it is incumbent upon organizations to be vigilant about the confidentiality of their submissions. This extends to maintaining the privacy of sensitive information within the QMS and being responsible for the data shared in regulatory interactions.
By comprehending the complexities of 21 CFR Part 812 and incorporating these crucial elements into their QMS, sponsors, investigators, and research organizations can guarantee that their activities not only adhere to regulations but are also positioned to improve patient outcomes and contribute to the progress of medical science.
Common Challenges and Best Practices for IDE Compliance
Ensuring adherence to the Code of Federal Regulations Title 21, Part 812 presents a multifaceted challenge, akin to the stringent compliance needs seen in the banking sector and advanced IT companies. In the field of medical investigations, issues range from intricately designed studies to precise patient recruitment strategies, rigorous data management, and meticulous regulatory inspections. To navigate these complexities, sponsors and investigators must adopt best practices that mirror the commitment to excellence demonstrated by organizations such as M&T Bank in maintaining software quality, or Scieneers with their robust data engineering services. For instance, adopting stringent Clean Code standards and leveraging advanced features of IDEs, like the comprehensive refactoring capability of PyCharm, can lead to improved study maintainability and reliability. Furthermore, in line with the FDA's focus on clear, conspicuous, and neutral communication of prescription drug information, trials must present data and regulatory submissions with equal clarity and neutrality to foster trust and ensure compliance. By integrating such best practices, sponsors and investigators can significantly improve their prospects for successful Investigational Device Exemption (IDE) compliance, akin to how software standards safeguard the highly sensitive data in banking. This structured approach to clinical trial management not only aligns with the FDA's public health mission but also upholds the integrity of clinical research in the quest for medical advancements.
Conclusion
In conclusion, 21 CFR Part 812 is a crucial regulatory framework governing clinical investigations involving investigational devices. It outlines the responsibilities of sponsors, investigators, and institutional review boards (IRBs) to ensure device safety and efficacy.
Key components of 21 CFR Part 812 include the IDE application and approval process, differences between significant risk and nonsignificant risk devices, exemptions from IDE requirements, labeling and distribution protocols, informed consent and subject protection, monitoring and reporting responsibilities, and quality management systems (QMS) and compliance.
Compliance with these regulations is essential for conducting safe and effective clinical investigations. Understanding IDE applications, adhering to labeling and distribution requirements, and prioritizing informed consent and subject protection are crucial. Additionally, maintaining rigorous monitoring and reporting practices, implementing a robust QMS, and adopting best practices enhance compliance and contribute to the advancement of medical science.
By adhering to 21 CFR Part 812, sponsors and investigators can conduct clinical investigations that prioritize patient safety and contribute to medical advancements. These regulations provide a framework for ethical and scientific conduct in clinical trials, ensuring the safety of participants and the integrity of the research process.
In summary, understanding and adhering to the key components of 21 CFR Part 812 are essential for sponsors and investigators to conduct compliant and ethical clinical investigations. By prioritizing patient safety and adhering to these regulations, stakeholders can contribute to the advancement of medical science and improve patient outcomes.




