Introduction
The 510(k) submission process is a critical step for medical device manufacturers seeking market clearance from the U.S. Food and Drug Administration (FDA). This rigorous pathway involves comparing the new device to an existing, legally marketed predicate device to demonstrate its safety and effectiveness. Manufacturers must thoroughly understand the subject device, its users, and the instructions for use, as well as analyze the competitive landscape to identify a suitable predicate device.
Additionally, industry news and evolving regulations highlight the dynamic nature of this process. The 510(k) submission serves the purpose of ensuring patient safety while fostering innovation. In this article, we will explore the types of 510(k) submissions, the step-by-step submission process, common challenges, and tips for success.
By delving into these details, manufacturers can navigate the intricate journey of the 510(k) submission process more effectively.
Understanding 510(k) and Its Purpose
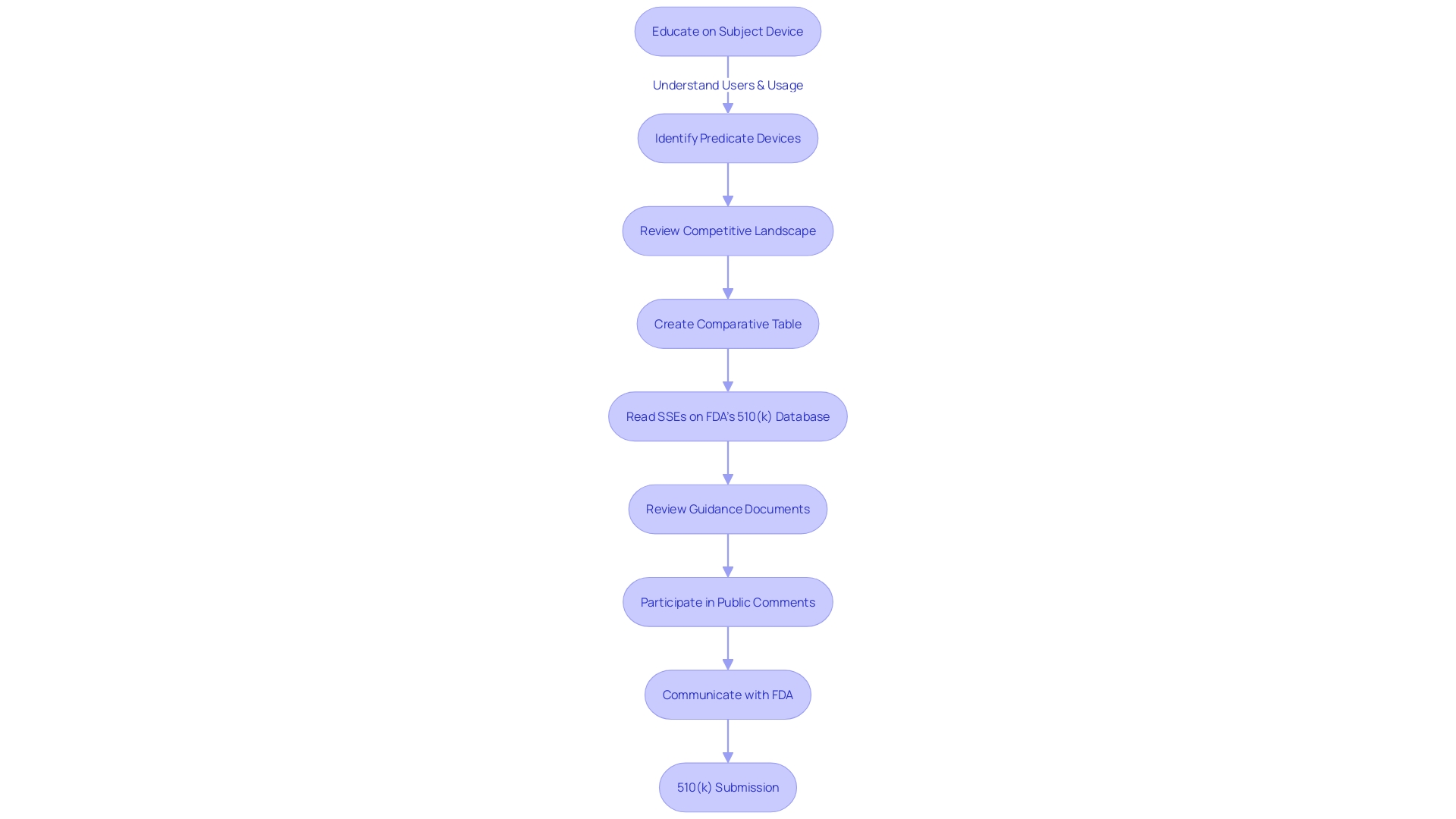
Navigating the 510(k) submission process is a crucial step for manufacturers aiming for market clearance by the U.S. Food and Drug Administration (FDA). This demanding pathway requires the new equipment to be compared to an existing, legally marketed precedent product. The comparison shows that the new equipment is substantially equivalent in terms of safety and effectiveness. To accomplish this, manufacturers must fully engage in comprehending the subject equipment, its users, and the instructions for use, including any warnings and cautions. A comprehensive examination of the competitive landscape is also crucial, which entails reviewing a variety of materials such as research literature, clinical studies, and marketing materials to identify a precedent product with the same intended purpose and technological features.
A comparative table is then created to compare the new apparatus with the predicate, emphasizing both similarities and differences. It is also essential to consult the FDA’s 510(k) database for Summaries of Safety and Effectiveness to further inform the comparison. This aspect of preparation is underscored by the FDA's modernization efforts, which have led to the development of a draft guidance document released on September 7, 2023, detailing best practices for predicate selection.
Moreover, industry news, such as the recent comprehensive rules for AI regulation released by the Biden administration and ongoing discussions on the draft AI Act in Europe, reflects the dynamic landscape in which these regulatory processes operate. It is in this framework that the 510(k) serves its function: to maintain patient safety while promoting innovation, ensuring that new medical products entering the market are assessed against established standards of safety and functionality.
Types of 510(k) Submissions
The 510(k) FDA approval process is a critical pathway for medical instrument manufacturers to bring their products to market. It includes three primary categories of applications: Classic, Condensed, and Special 510(k). The Traditional 510(k) is the most common route, requiring a comprehensive comparison with a legally marketed predicate instrument. This type of submission is necessary when an equipment is significantly similar to an existing product but has minor modifications. The Abbreviated 510(k) leverages guidance documents, special controls, and recognized standards to streamline the review process, suitable for products where a consensus standard exists. The Special 510(k) is for manufacturers making slight modifications to their own FDA-cleared products, where the changes do not affect safety or effectiveness.
To successfully navigate this process, it is essential to have a comprehensive understanding of the equipment, including its intended users, usage instructions, warnings, and cautions. This knowledge, often gathered in collaboration with Marketing departments, informs a comparative analysis against competitor products. By examining research literature, clinical studies, and relevant commercial materials, manufacturers can identify suitable precedent products and create comparative tables to demonstrate similarities and differences.
Moreover, the Summaries of Safety and Effectiveness Data (Seeds) available in the FDA's 510(k) database offer valuable insights into precedent regulatory decisions and help in evaluating the adequacy of substantiating equipment equivalence. Comprehending the intricacies of each type of application and creating a strong 510(k) application are crucial to minimize risks of non-compliance and guarantee a more streamlined regulatory review process.
Step-by-Step 510(k) Submission Process
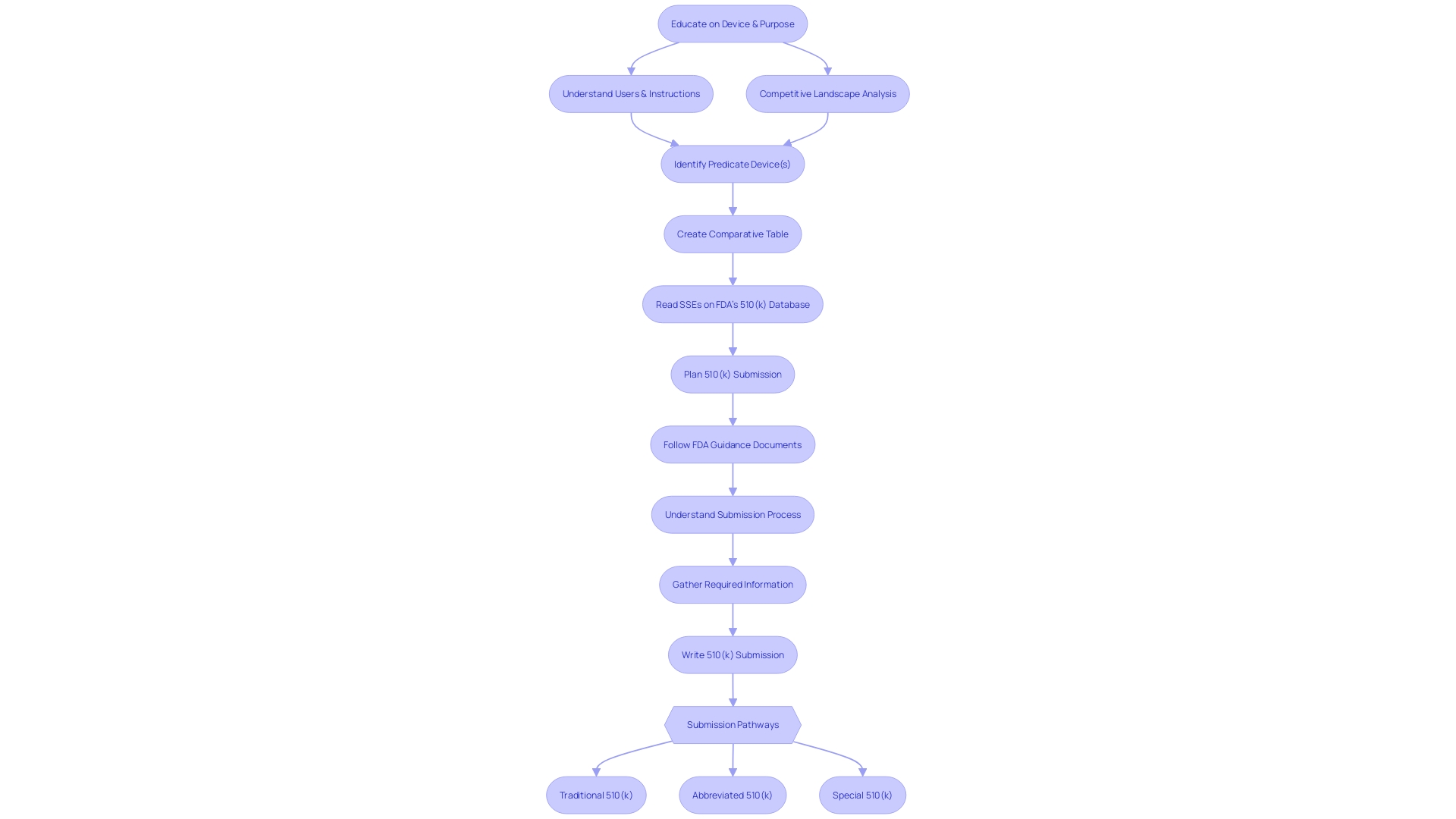
The 510(k) submission process is a meticulous journey that medical instrument manufacturers embark upon to achieve FDA clearance. It begins with a comprehensive understanding of the apparatus and its objective, taking into account users such as clinicians, physicians, dentists, and patients, as well as thoroughly reviewing the instructions for use, including any warnings and cautions. This fundamental knowledge is crucial as expressed by industry professionals, highlighting the importance of a thorough comprehension of the apparatus, its users, and the competitive environment.
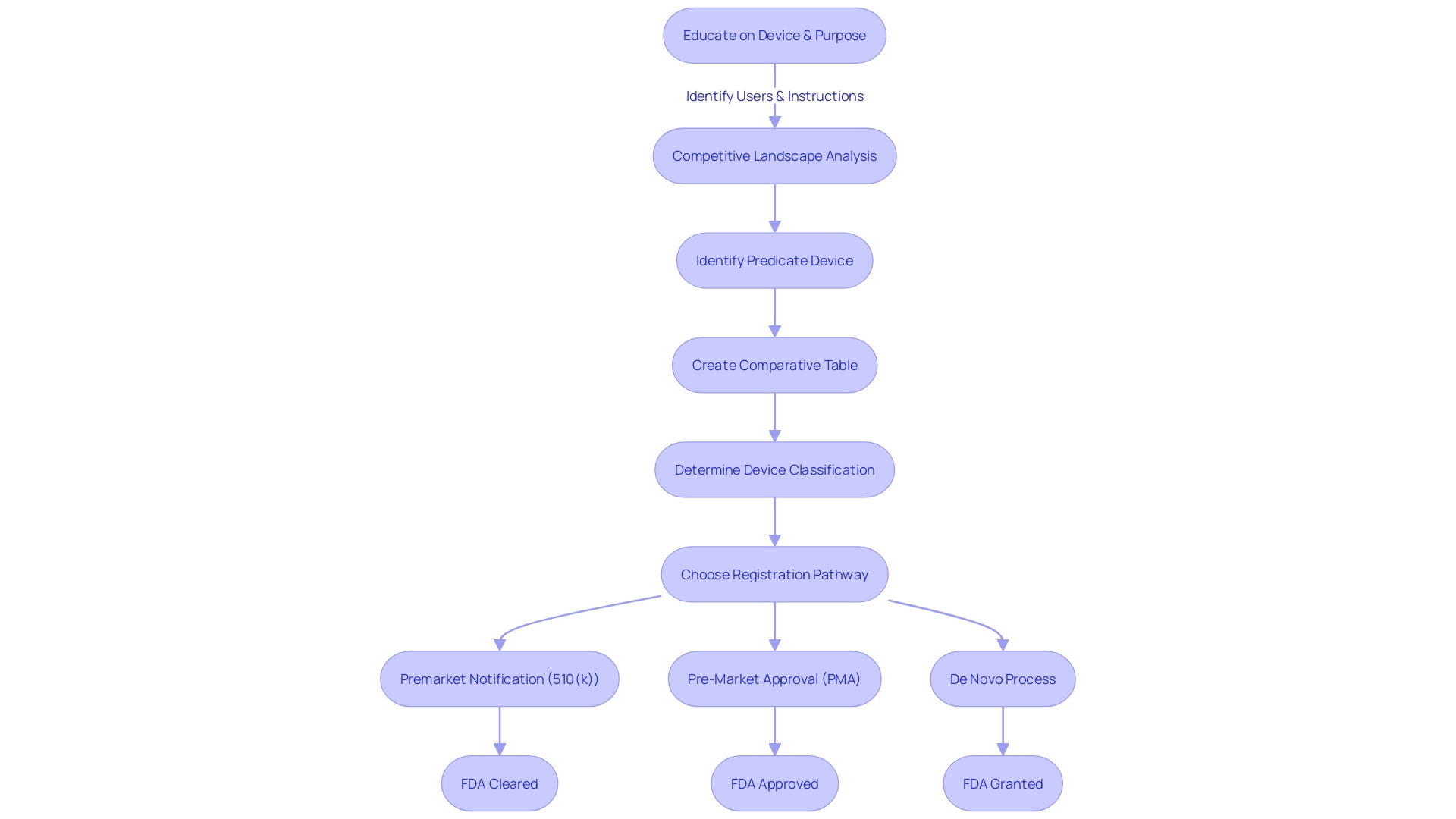
Afterwards, manufacturers must identify a suitable reference product with the same intended use and similar technological characteristics. This process involves an extensive review of research literature, clinical studies, and competitor information to construct a comparative table that supports their submission. Since the FDA classifies medical equipment into three levels of classification based on patient risk, determining the accurate categorization is a crucial step that determines the registration pathway to be followed.
Whether it's a Premarket Notification (510(k)), Pre-Market Approval (PMA), or De Novo process, the goal is to ensure that the device is FDA Cleared, Approved, or in the case of the De Novo process, Granted. The terminology used within the industry—Registered, Cleared, Approved, and Granted—often leads to confusion, but each term represents a distinct regulatory status that manufacturers must clearly understand.
The 510(k) process itself involves compiling and reviewing a vast amount of information from various sources within a company, such as experiment reports and meeting notes. This phase is crucial, as emphasized by the experiences of the founders of Artos, who faced the intricacies of regulatory filings firsthand. The document must be presented in a format that meets FDA's rigorous standards, which can extend the timeline by weeks or months.
Moreover, the recent FDA final rule for direct-to-consumer prescription drug advertisements highlights the significance of clarity and simplicity in presenting information, which is equally relevant to the 510(k) process. The documentation must use consumer-friendly language and terms, ensuring that the information is easily comprehensible to both the reviewers at the FDA and the stakeholders it will impact.
To summarize, the 510(k) process is a structured yet demanding pathway that requires a thorough understanding of the product, precise classification, and a well-prepared application that complies with FDA's standards. As companies like Medtronic continue to innovate and deliver groundbreaking technologies, the insights and experiences of both established healthcare leaders and emerging entrepreneurs shed light on the intricacies of navigating this process successfully.
Common Challenges and Tips for Success
Exploring the 510(k) process is a complex journey filled with possible obstacles that can hinder even experienced manufacturers. To effectively chart this course, a manufacturer must first become intimately familiar with the device in question, comprehensively understanding its users, including clinicians and patients, and meticulously studying the instructions for use, paying close attention to warnings and cautions. This knowledge is fundamental; without it, the project could miss the mark from the beginning.
The competitive landscape offers a wealth of insights that should not be overlooked. Collaborating with Marketing teams to review research literature, clinical studies, and competitor materials such as websites, brochures, and labeling can illuminate the path to identifying appropriate predicate devices—those with similar intended use and technological characteristics. A comparative table becomes an invaluable tool here, crystallizing the similarities and differences that will be pivotal in the FDA's assessment of safety and effectiveness.
In September 2023, the FDA revealed draft guidance reflecting a modernized approach to selecting predicates for 510(k) filings. This guidance was a direct result of analyzing public feedback on the use of older predicates and recognizing the value in their long-term safety data. The guidance outlines best practices, emphasizing the importance of selecting a legally marketed product and ensuring that any technological differences do not raise new safety and effectiveness concerns.
Public comments play a significant role in shaping regulatory guidance and manufacturers are encouraged to participate in this dialogue. It's essential to be mindful of confidentiality when submitting comments electronically, as they become part of the public record. Navigating the fine line between transparency and protecting sensitive information is a critical skill for manufacturers engaging with the FDA.
Ultimately, success in the 510(k) submission process is predicated on a thorough understanding of the device and its competitive environment, and the ability to effectively communicate this information to the FDA. By harnessing the power of detailed preparation and leveraging resources like the FDA’s 510(k) database to examine Summaries of Safety and Effectiveness, manufacturers can enhance their chances of achieving a favorable review outcome.
Conclusion
In conclusion, the 510(k) submission process is a critical step for medical device manufacturers seeking market clearance from the FDA. It involves comparing the new device to an existing predicate device to demonstrate its safety and effectiveness. Manufacturers must thoroughly understand the device, its users, and the instructions for use, as well as analyze the competitive landscape to identify a suitable predicate device.
There are three main types of 510(k) submissions: Traditional, Abbreviated, and Special 510(k). Each type has its own requirements, and manufacturers must have a thorough understanding of the device and its users to select the appropriate submission type. The FDA's 510(k) database provides valuable insights into past regulatory decisions.
The step-by-step 510(k) submission process involves understanding the device and its users, identifying a suitable predicate device, and compiling a well-prepared submission. The documentation must meet the FDA's standards and use consumer-friendly language for clarity.
Common challenges in the process include comprehensive device knowledge, navigating the competitive landscape, and understanding evolving guidance and regulations. Manufacturers should collaborate with marketing teams, review competitor materials, and stay informed about the latest guidance and best practices. Public comments also play a significant role in shaping regulatory guidance.
In summary, the 510(k) submission process requires a comprehensive understanding of the device, accurate classification, and a well-prepared submission. By preparing thoroughly and leveraging available resources, manufacturers can enhance their chances of achieving a favorable review outcome and bringing their innovative medical devices to market while ensuring patient safety.




