Introduction
Biocompatibility testing plays a crucial role in ensuring the safety and efficacy of medical devices. Governed by a complex regulatory framework, including standards set by entities like the FDA and ISO, these tests evaluate how devices interact with the human body and identify any potential adverse effects. Adhering to these standards is not only about compliance but also about fostering innovation and ensuring the availability of safe and effective medical technologies.
This article explores the regulatory landscape, FDA guidance, ISO standards, material selection, key concepts in biocompatibility testing, the role of accreditation, best practices, and common challenges in conducting these tests. By understanding and navigating this complex environment, medical device manufacturers can protect patient health and bring forward innovative technologies.
Regulatory Framework and Standards
Evaluating the compatibility of healthcare instruments is a vital part of guaranteeing the well-being of the patient and the effectiveness of the product. It is governed by a complex framework of standards and regulations, notably by entities such as the U.S. Food and Drug Administration (FDA) and the International Organization for Standardization (ISO). These instructions are crucial in preserving the authenticity of healthcare equipment throughout their lifespan.
In the realm of healthcare instrument control, access to the mechanism's Bill of Materials (BOM) is compulsory. With regulations evolving and varying across different markets, medical device manufacturers must maintain a comprehensive understanding of their product's reach. Some regulations are specific to particular countries or regions, and manufacturers with a global market presence often follow the highest level of requirement to ensure compliance across all markets.
The significance of following voluntary consensus guidelines is emphasized by organizations like the Standards Development Organizations (SDOs). These criteria are established on principles of transparency, openness, balance, and due process, ensuring that a wide range of stakeholders can contribute to the creation of these guidelines. These standards are not only about compliance but also about fostering innovation and ensuring the availability of safe and effective technologies.
The ever-changing nature of regulation in the field of healthcare is demonstrated by recent policy intent statements made by regulatory bodies such as the Medicines and Healthcare products Regulatory Agency (MHRA). Such statements outline the intention to acknowledge approvals of healthcare instruments from other regulatory regions, thereby simplifying the market access for manufacturers and ensuring that patients have access to innovative and safe health technologies.
For manufacturers of healthcare equipment, it is crucial to not only comprehend the current regulatory environment but to be ready for future changes and advancements. The integration of AI-driven regulatory platforms and international collaboration on standards can greatly assist in navigating this complex environment.
In the end, the objective is to guarantee that healthcare equipment fulfills rigorous safety standards, thereby safeguarding patient well-being and bolstering the sector in advancing the most groundbreaking and efficient technologies.
Overview of FDA Guidance on Biocompatibility Testing
Biocompatibility evaluation for medical equipment is a crucial element of the FDA's regulatory structure, which aims to guarantee the safety, efficiency, and security of such equipment. These tests are designed to assess how an apparatus interacts with the human body and to identify any potential adverse effects. The FDA's guidance, while not legally enforceable, serves as a compass for manufacturers to align their protocols with current scientific and regulatory standards. Compliance with these guidelines is vital for manufacturers aiming to secure FDA approval.
Considering the crucial function that healthcare instruments fulfill in patient care, the FDA highlights the significance of thorough compliance assessment, which includes activities such as sampling, testing, and certification. This ensures that an apparatus fulfills the particular prerequisites delineated by consensus standards—formulated with the concepts of openness, stakeholder involvement, and proper procedure in consideration. Such standards are vital to preserving the quality and safety of devices, advancing innovation, and facilitating global harmonization.
Manufacturers must be meticulous in documenting their biocompatibility testing processes, as this documentation is a key element in the regulatory submission. The FDA provides a framework for the submission of this evaluation, detailing the information that will be made publicly available on the agency's website. The agency's approach to standardization and its guidance documents reflect a commitment to public health and the advancement of healthcare technology.
Significantly, the recent draft guidance from the FDA aims to clarify the implementation of the Breakthrough Devices Program, providing a pathway for innovative instruments that offer a more efficient approach to treating or diagnosing life-threatening conditions. This initiative highlights the FDA's commitment to promoting advancement while guaranteeing the safety and effectiveness of healthcare equipment. Truly, the organization's supervision is a vital component of public health, as evidenced by data indicating that healthcare instruments were associated with more than 1.7 million injuries and 83,000 deaths in the United States over a ten-year period. Such figures highlight the imperative of stringent biocompatibility testing and regulatory compliance.
Understanding ISO 10993 and Its Application
'ISO 10993 guidelines play a crucial role in guaranteeing the compatibility and security of healthcare instruments.'. This collection of principles provides a thorough structure for assessing the biological reactions of instruments, spanning from basic tools to intricate implants and biologic substances. Given the wide variety of equipment classified into over 7,000 groups by the World Health Organization, the versatility and inert nature of materials like silicone underscore the critical significance of these guidelines.
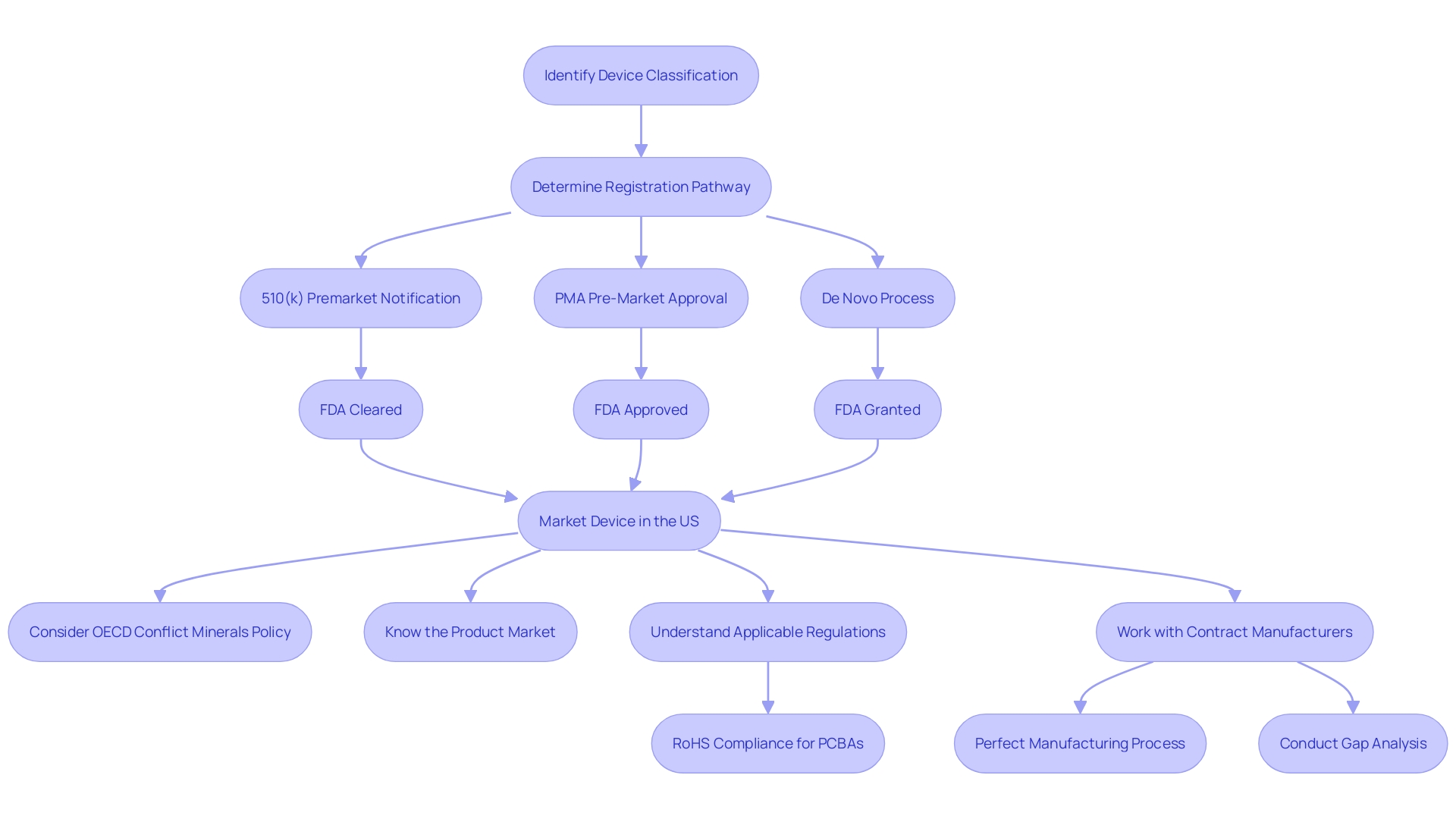
The evaluation of biocompatibility is not a static process but an ongoing one, requiring a deep understanding of the product's lifecycle, market reach, and the materials used in its construction. Companies must adopt a global perspective on regulatory compliance to navigate the varied landscape of international regulations, as pointed out by the OECD Conflict Minerals policy. This approach is essential, especially in complex, multi-tiered supply chains that can span continents and involve numerous stakeholders.
Recent advancements, like the establishment of cutting-edge examination centers by UL Solutions in Michigan, have emphasized the state's significance in medical instrument technology. These facilities are equipped to perform a range of tests according to manufacturer specifications and adapt quickly to emerging needs. Such advancements play a critical role in addressing risks related to quality, safety, and cybersecurity, while also fostering innovation.
The utilization of consensus norms created by Standards Development Organizations (SDOs) is essential to a strong regulatory framework. These guidelines, which are clear, accessible to involvement, and fair, are crucial for upholding regulatory excellence and promoting the worldwide alignment of product evaluation.
Incorporating these benchmarks into biocompatibility evaluation guarantees a trustworthy conformity assessment, which involves sampling, evaluation, and certification processes. This commitment to high standards is not only a regulatory necessity but also a dedication to patient safety and innovation in the healthcare industry.
Material Selection and Sample Preparation for Biocompatibility Testing
The complexities of biocompatibility testing and evaluation in the extensive field of healthcare technology are essential. With over two million varieties of healthcare instruments classified into more than 7,000 clusters, the choice of materials becomes a intricate yet crucial decision. Among the diverse materials, such as metals, thermoplastics, and ceramics, silicone distinguishes itself with its versatility, excellent physical properties, and resistance to chemical and thermal degradation. Its chemical inertness is a key factor in its widespread use and biocompatibility, making it a preferred choice for various applications in healthcare technology.
When choosing materials for healthcare tools, it is crucial to take into account their chemical composition, mechanical characteristics, and interaction with the human body. The suitability of a material for the intended application must be meticulously evaluated, balancing its inherent properties with the potential effects on patients. For instance, the evolution of silicone from coating glassware and needles to a wide array of medical-grade materials exemplifies the need for customized solutions that meet the stringent requirements of modern healthcare.
Preparation of samples for biocompatibility evaluation, which involves sterilization methods and considerations for both laboratory and animal experiments, plays a fundamental role in ensuring the safety and effectiveness of healthcare instruments. Conformity assessment methods such as sampling, inspection, and certification are integral to a robust regulatory framework, adhering to principles of transparency and stakeholder participation as outlined by Standards Development Organizations (SDOs).
Recent advancements, as highlighted by Dr. Frank Shellock, have shown how innovative materials can significantly enhance patient outcomes, such as the Flora SmoothSilk Tissue Expander's MR Conditional technology, which allows patients to undergo critical MRI diagnostics during breast reconstruction. This highlights the significance of ongoing innovation and uniformity in healthcare technology, promoting patient access to new equipment that meets strict safety criteria.
In summary, the careful choice of materials and comprehensive sample preparation procedures are crucial stages in the biocompatibility assessment and evaluation of healthcare equipment, in accordance with ISO 10993 regulations. This guarantees that healthcare equipment is secure, efficient, and has a positive impact on patient care.
Key Concepts in Biocompatibility Testing: Cytotoxicity, Irritation, and Sensitization
Biocompatibility evaluation assesses the possible influence of healthcare instruments on biological systems, with a particular emphasis on the interaction of these instruments with the human body. This form of testing is crucial in determining whether a medical instrument is suitable for its intended use without causing harm. Key biocompatibility tests include evaluations of cytotoxicity, irritation, and sensitization, which are crucial for ensuring the safety and effectiveness of the equipment.
Cytotoxicity tests are designed to evaluate whether an object or its components release substances that may be toxic to cells. This is a crucial test as it provides an early indication of potential problems that could arise from direct or indirect contact with the equipment.
Irritation studies are carried out to determine if materials used in medical equipment cause irritation to tissues upon contact. This is especially crucial for gadgets that interact with skin or internal tissues during their utilization.
Sensitization testing is crucial for identifying the potential for an allergic reaction due to repeated exposure to a material. As allergies can have serious consequences for patients, comprehending and reducing sensitization risks is a priority in the advancement and regulatory approval of healthcare equipment.
The approaches for these tests are well-established and conform to international guidelines, such as those outlined by the International Organization for Standardization (ISO) in the ISO 10993 series of guidelines. These guidelines offer a structure for the assessment and experimentation of healthcare equipment concerning their physiological impacts.
By implementing thorough conformity evaluation processes, as described by the Office of Management and Budget (OMB) and Standards Development Organizations (SDOs), guarantees that medical instruments fulfill the utmost quality and safety criteria. The adherence to transparency, openness, balance of representation, and due process is essential in the development of consensus standards that form the backbone of regulatory quality.
Medical manufacturers must also maintain visibility into their supply chains and the materials used in their products. Complete oversight into the Bill of Materials (BOM), including component serialization and lot traceability, is a regulatory necessity and an important risk management strategy. As the healthcare equipment industry continues to advance and grow, facilities such as UL Solutions' laboratory in Rochester Hills, Michigan, are equipped to offer comprehensive assessment capabilities to meet the increasing need for biocompatibility evaluation and address potential risks in quality, safety, and cybersecurity, while supporting the rapid rate of healthcare equipment innovation.
Biocompatibility Testing for Specific Medical Device Materials
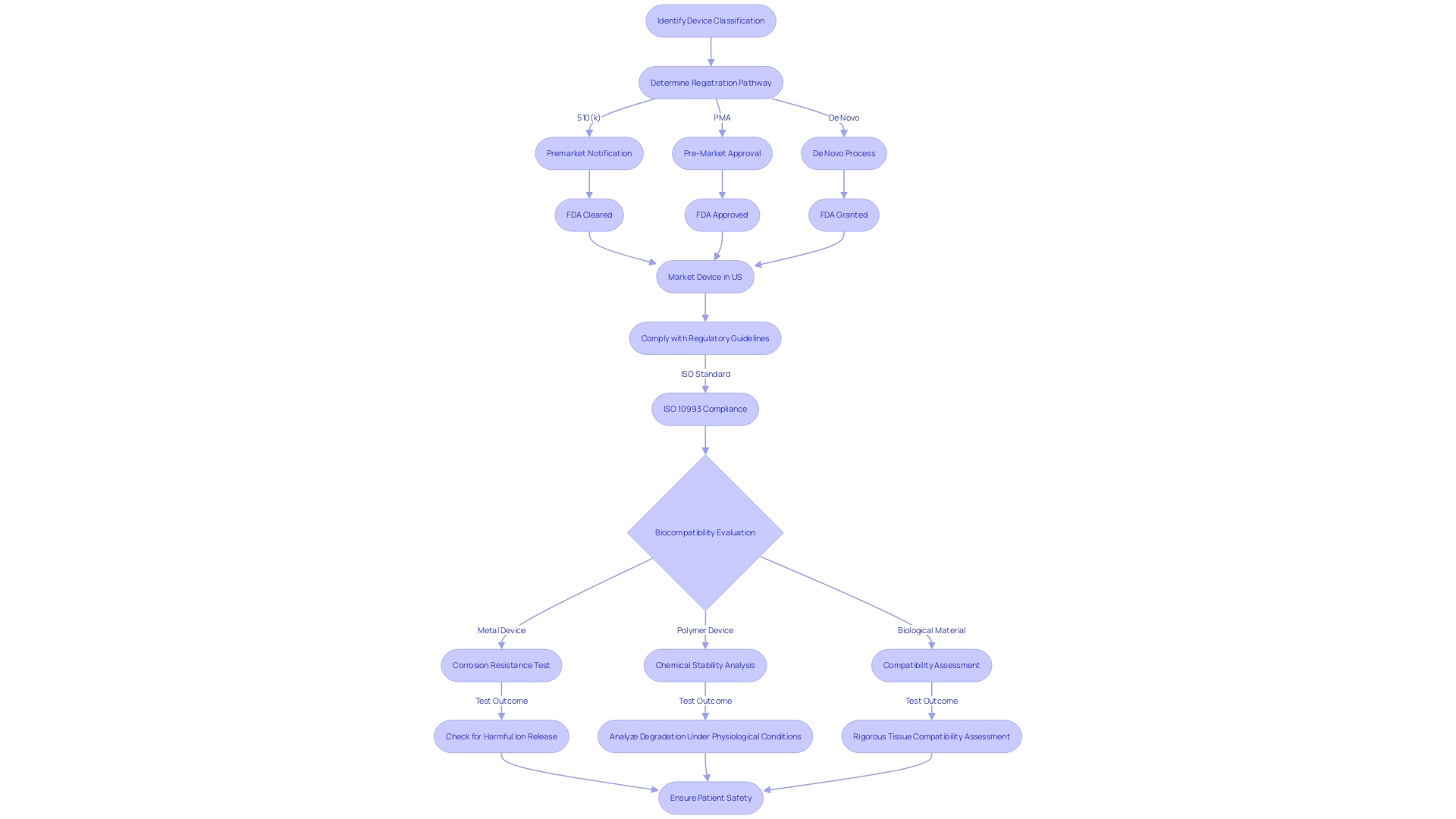
Biocompatibility evaluation for medical devices is a complex process that must be tailored to the material composition of each device. Metals, polymers, ceramics, and biological materials each have distinctive characteristics that affect their interaction with the human body, thus requiring customized evaluation protocols.
Metals, for instance, are commonly used in implants and must be tested for corrosion resistance and potential release of harmful ions. Polymers are favored for their flexibility and range of applications, from tubing to prosthetics, and require analysis for chemical stability and degradation under physiological conditions. Evaluation for these materials focuses on their brittleness and wear resistance. Lastly, biological materials, which are used in applications like tissue engineering, necessitate rigorous assessment of their compatibility with living tissues, including potential immunogenic responses.
The intricacy of biocompatibility evaluation is emphasized by the variety of materials utilized in healthcare instruments and emphasizes the significance of complying with regulatory guidelines, such as ISO 10993, to guarantee patient safety and instrument effectiveness. With the continuous advancement of healthcare technology, the field of assessing biocompatibility also evolves, always with the goal of safeguarding patient well-being.
The Role of Accreditation and Assessment in Biocompatibility Testing
Accreditations and assessments are crucial in establishing the quality and reliability of biocompatibility tests for healthcare instruments. Biocompatibility evaluation is a thorough process that assesses whether a medical device is compatible with human tissue and bodily systems. It is crucial that such testing is performed by laboratories that conform to the highest levels of quality management systems. Accredited laboratories are rigorously audited by third parties to ensure impartiality and technical competence, as well as adherence to EU regulations and international standards, including ISO 10993.
The process of accreditation involves a thorough review of the lab's ability to carry out specific tests, assessing both the safety management systems in place and the technical proficiency of the lab to provide accurate results. For example, within the European Quality Infrastructure ecosystem, accreditation ensures the independence and competence of conformity assessment bodies. This is critical because, in healthcare, inaccurate test results can have life-or-death consequences, such as in cancer treatment where laboratory test results directly influence therapy decisions.
In the context of healthcare tools, it is not only the quality of production processes that is important, but also the safety and effectiveness of the products themselves. This is where the role of National Accreditation Bodies becomes crucial. They evaluate the fairness and technical ability of Conformity Assessment Bodies, guaranteeing that healthcare equipment satisfies the rigorous prerequisites needed for market access.
Additionally, the recent expansion of UL Solutions’ laboratory in Rochester Hills, Michigan, highlights the increasing demand for such accredited evaluation services. The lab's state-of-the-art capabilities enable a variety of testing techniques, meeting the specific requirements of manufacturers in the healthcare industry. It also emphasizes the importance of dealing with possible hazards such as quality, safety, and cybersecurity concerns while promoting creativity and safeguarding end-users of healthcare equipment.
Therefore, collaborating with authorized laboratories is not only about following regulations, but it is also a dedication to ensuring patient safety and ensuring the quality of products, which ultimately fosters innovation and builds trust within the industry.
Best Practices for Conducting Biocompatibility Tests
Biocompatibility testing is a critical aspect of medical instrument development, ensuring that the products are safe and fit for their intended use. When planning biocompatibility studies, various important factors must be considered, such as the materials employed, material processing techniques, manufacturing methods, and the intended clinical application. The intended anatomical location and the duration of exposure to the instrument are also pivotal factors, as they directly influence the potential for tissue responses.
For an accurate assessment of biocompatibility risks, it's not only chemical toxicity that must be evaluated. Physical characteristics of the equipment, such as geometry, mechanical, thermal, and electromagnetic forces on surrounding tissues, and surface properties, play a significant role in how the apparatus interacts with the body. The FDA underscores the importance of monitoring any changes in manufacturing and processing, as these can significantly impact biocompatibility. To this end, manufacturers are advised to draw upon a range of information sources, including prior experiences with similar materials, data from other manufacturers, supplier details, and published literature.
Regarding analytical chemistry testing, the FDA provides guidance that covers the techniques for evaluating biocompatibility of devices used in healthcare. This includes chemical characterization, which can be instrumental in developing a comprehensive biocompatibility strategy. Nevertheless, it's important to acknowledge that approaches may differ depending on the particular healthcare apparatus inquiring, the substances involved, or pre-established industry methods. Device-specific FDA guidances or acknowledged consensus criteria should always be followed where relevant.
Additionally, the device industry is backed by a wide range of services, including evaluation and software enhancement, which are crucial for adherence to regulatory standards. Companies that offer these services are essential for ensuring quality, facilitating audits, and ultimately, the safety of healthcare products. Keeping updated on the most recent advancements and utilizing the knowledge of industry authorities can be extremely valuable for companies aiming to navigate the intricacies of biocompatibility evaluation.
Common Challenges and Solutions in Biocompatibility Testing
Understanding the complexities of biocompatibility assessments for medical equipment is essential for guaranteeing patient well-being and fulfilling regulatory standards. The FDA's draft guidance document FDA-2024-D-4165 highlights the importance of analytical chemistry testing as a component of biocompatibility evaluation. Chemical characterization, while just one facet of a comprehensive biocompatibility strategy, must be customized to the distinct composition of each apparatus, taking into account any specific FDA guidances or recognized standards that may apply.
Adherence to the Organization for Economic Cooperation and Development's (OECD) Conflict Minerals policy underlines the need for comprehensive due diligence in material sourcing, especially for vital minerals like tin, tantalum, and tungsten. These practices are not only ethically necessary but also have a crucial role in upholding compliance and ensuring the safety of healthcare equipment.
The recent case study involving Cardinal Health illustrates the complexities of regulatory submissions, emphasizing the importance of a well-devised regulatory strategy for investigational drug applications. Likewise, manufacturers must maintain visibility into their product's Bill of Materials (BOM) and ensure their supply chain's compliance with current regulations.
'UL Solutions' new laboratory in Rochester Hills, Michigan, now meets the requirements of the healthcare device industry's need for comprehensive examination methods. This facility is designed to accommodate different specifications and address risks related to quality, safety, and cybersecurity, while also fostering innovation.
Despite the dependence on animal experimentation in preclinical trials, with an astonishing 90% of drugs failing in clinical trials after animal experimentation, the healthcare community is encouraged to reassess the effectiveness and ethics of current models and investigate more precise and economically feasible alternatives.
Risk management expert Bijan Elahi's teachings, encapsulated in his book 'Safety Risk Management for Medical Devices - Second Edition,' offer valuable insights into the principles of risk management in the medical device domain. His experience and practical advice can empower manufacturers to enhance their safety risk management strategies.
Ultimately, overcoming the challenges of biocompatibility testing requires a multifaceted approach, involving up-to-date knowledge of regulations, strategic material sourcing, thorough chemical characterization, and robust risk management practices.
Conclusion
In conclusion, biocompatibility testing is crucial for ensuring the safety and efficacy of medical devices. Adhering to FDA and ISO standards is essential for manufacturers to protect patient health and bring forward innovative technologies. Understanding the regulatory landscape and navigating it effectively allows manufacturers to ensure compliance and foster innovation.
ISO 10993 standards provide a comprehensive framework for evaluating biocompatibility and safety. Material selection plays a critical role, with silicone being widely used due to its versatility and biocompatibility. Sample preparation and conformity assessment methods are key for robust testing.
Key concepts in biocompatibility testing, such as cytotoxicity, irritation, and sensitization, are essential for evaluating the impact of devices on biological systems. Accreditation and assessment ensure the quality and reliability of tests, adhering to international standards.
Best practices include considering materials, manufacturing techniques, intended use, and exposure duration. Analytical chemistry testing and staying informed are vital. Overcoming challenges involves tailoring testing to device composition, due diligence in material sourcing, maintaining visibility into the Bill of Materials, and exploring alternatives to animal testing.
In conclusion, by understanding and navigating the complex environment of biocompatibility testing, manufacturers can protect patient health and bring forward innovative technologies. Adhering to regulatory standards, selecting appropriate materials, following best practices, and leveraging accredited laboratories are key steps in ensuring the safety and efficacy of medical devices.




