Introduction
Comprehensive clinical data management is crucial for the integrity of clinical trials and the assessment of the safety and efficacy of medical devices. With over two million types of medical devices in circulation globally, the importance of rigorous clinical data management cannot be overstated. This article explores the objectives, roles and responsibilities, phases, tools and systems, key features, benefits, and best practices of clinical data management.
From the integration of technology solutions to the evolving field of informatics, this article dives into the complexities and advancements in managing clinical trial data. Whether it's the meticulous handling of data or the utilization of electronic data management systems, the focus remains on ensuring accuracy, data integrity, and patient safety. Join us as we delve into the world of clinical data management and its pivotal role in advancing medical knowledge and patient care.
What is Clinical Data Management?
Ensuring the integrity of trials, which play a crucial role in evaluating the safety and effectiveness of medical devices, requires a comprehensive approach to handling and overseeing the relevant information. The World Health Organization estimates that with over two million types of medical devices in circulation globally, the vast majority of individuals will come across these devices during their lifetime, emphasizing the significance of thorough management of medical information. The European Union Medical Device Regulation (EU MDR) highlights this by stating that investigations must prioritize the rights, safety, dignity, and well-being of subjects, ensuring that the resulting data is scientifically robust and reliable.
MedTech companies, especially those producing high-risk devices, understand that conducting experiments is not only essential to bringing devices to market but also for maintaining market presence. Based on the 2024 State of the MedTech Industry Benchmark Report, medical activities are highly prioritized in the industry. Clinical information systems act as the crucial foundation for handling the extensive quantities of information produced by these experiments, guaranteeing adherence to regulatory prerequisites and preserving information integrity.
In the United States, evidence from trials plays a significant role in regulatory submissions, with 10-15% of successful 510(k) submissions for Class II devices and all Class III devices relying on such information to demonstrate safety and effectiveness. The function of handling medical information extends beyond the mere collection of information; it encompasses a comprehensive process that includes the meticulous handling of records to produce reliable and high-quality data. This procedure is essential as it not only protects participant safety but also guarantees that the information is interpreted accurately and is considered credible by regulatory authorities.
The latest advancements in the handling of medical information have been influenced by the integration of technology solutions that enhance the user experience and patient safety. For instance, Universal Health Services has developed tools that integrate seamlessly with the Oracle Millennium EHR, enhancing workflows for nurses, physicians, and pharmacists across more than 85 hospitals. These advancements are a sign of the continuous progress in organizing and overseeing medical information, with the goal of enhancing the precision of information and the well-being of patients.
Objectives of Clinical Data Management
Ensuring the quality, integrity, and traceability of information throughout the duration of a medical experiment is crucial. It encompasses not only the verification of information accuracy and completeness but also the establishment of rigorous validation checks and the maintenance of a meticulous audit trail documenting all changes to the information. By utilizing a structured information quality framework, management systems for healthcare information provide essential tools for cleaning, discrepancy management, and reliable validation of information. These systems are designed to identify and resolve inconsistencies, uphold information standards, and ensure the fidelity of information representation, which is essential to informed decision-making and maintains the integrity of clinical research.
Experts in the field emphasize the significance of reliable patient information, questioning whether the industry acknowledges the extent of quality issues with the collected data and whether it can be confidently integrated into broader healthcare objectives. The yearly Healthcare Data Quality Survey carried out by Clinical Architecture assesses the perceived quality of healthcare information, its influence on different objectives, and the factors that contribute to its degradation across various market segments. This introspective inquiry reveals the industry's critical need for high-quality information to ensure patient safety and the efficacy of medical devices.
Actually, the World Health Organization calculates that more than two million varieties of medical devices are accessible on the market, with medical information acting as the basis for regulatory submissions. The European Union Medical Device Regulation emphasizes the importance of conducting investigations to prioritize the rights and safety of subjects, ensuring that scientific evidence is valid, reliable, and strong. The handling of this information is not only a regulatory necessity but also a crucial aspect of patient well-being and the pathway to market entry for medical devices, with a substantial proportion of Class II and all Class III devices undergoing trials to validate their safety and efficacy.
Moreover, the digital future of healthcare is being influenced by cutting-edge technologies like artificial intelligence, which, as recent news suggests, necessitate careful consideration of the information inputted into electronic health records. Biomedical researchers advocate for transparency and rigorous testing of healthcare algorithms to ensure their utility and accuracy, suggesting the involvement of federal agencies in the evaluation process. As healthcare continues to develop, the responsibility of handling medical information becomes more intricate and crucial, necessitating ongoing attention to uphold the utmost standards of information quality and reliability.
Roles and Responsibilities in Clinical Data Management
In the field of medical studies, the handling of information is a intricate and multifaceted responsibility, necessitating the skills of different specialized positions. These experts, such as information managers, database developers, biostatisticians, and quality control staff, collaborate to uphold the accuracy of research information. Data organizers coordinate the process of information control, guaranteeing that the gathering, handling, and reporting of medical information are carried out smoothly. Database programmers are responsible for developing and maintaining reliable clinical information management systems, which are essential for the secure handling of sensitive experimental information. Biostatisticians bring their analytical prowess to the table, meticulously analyzing the information to extract meaningful insights that drive forward medical advancements. Lastly, quality control personnel are the gatekeepers of accuracy and quality, a critical role that safeguards the validity of the outcomes.
Given the crucial importance of accurate information handling, emphasized by governing organizations like the European Union Medical Device Regulation (EU MDR), safeguarding participant rights and ensuring the dependability of research outcomes are of utmost importance. Recent updates to the International Council for Harmonization (ICH) E6 GCP guidelines, slated for implementation in late 2024, have brought attention to data governance, emphasizing the need for trials to prioritize participant safety and the integrity of information.
Furthermore, the field of informatics plays a transformative role in healthcare by enhancing the way information is generated, stored, processed, and utilized for decision-making. Clinical informatics, as a subspecialty, focuses on leveraging information and communication systems to optimize patient care, improve health outcomes, and fortify the clinician-patient relationship. The American Medical Association (AMA), American Nurses Association (ANA), and the American College of Obstetrics and Gynecology (ACOG) are among the many medical specialty societies that have acknowledged the critical role informatics plays in modern healthcare practices.
While we traverse the complex terrain of data administration, it is crucial to acknowledge the combined endeavors and personal inputs of those engaged in the administration of data. The Credit taxonomy serves as a standardized framework to attribute these contributions, echoing the sentiments of the Consortia Advancing Standards in Research Administration Information (CASRAI), which seeks to streamline the information flow in research. With the implementation of enhanced transparency regulations in the EU, like the revised Clinical Trials Information System (CTIS), interested parties now have earlier and more effective access to information regarding medical experiments, representing a considerable step towards increased transparency in the field of medical research.
Phases of Clinical Data Management
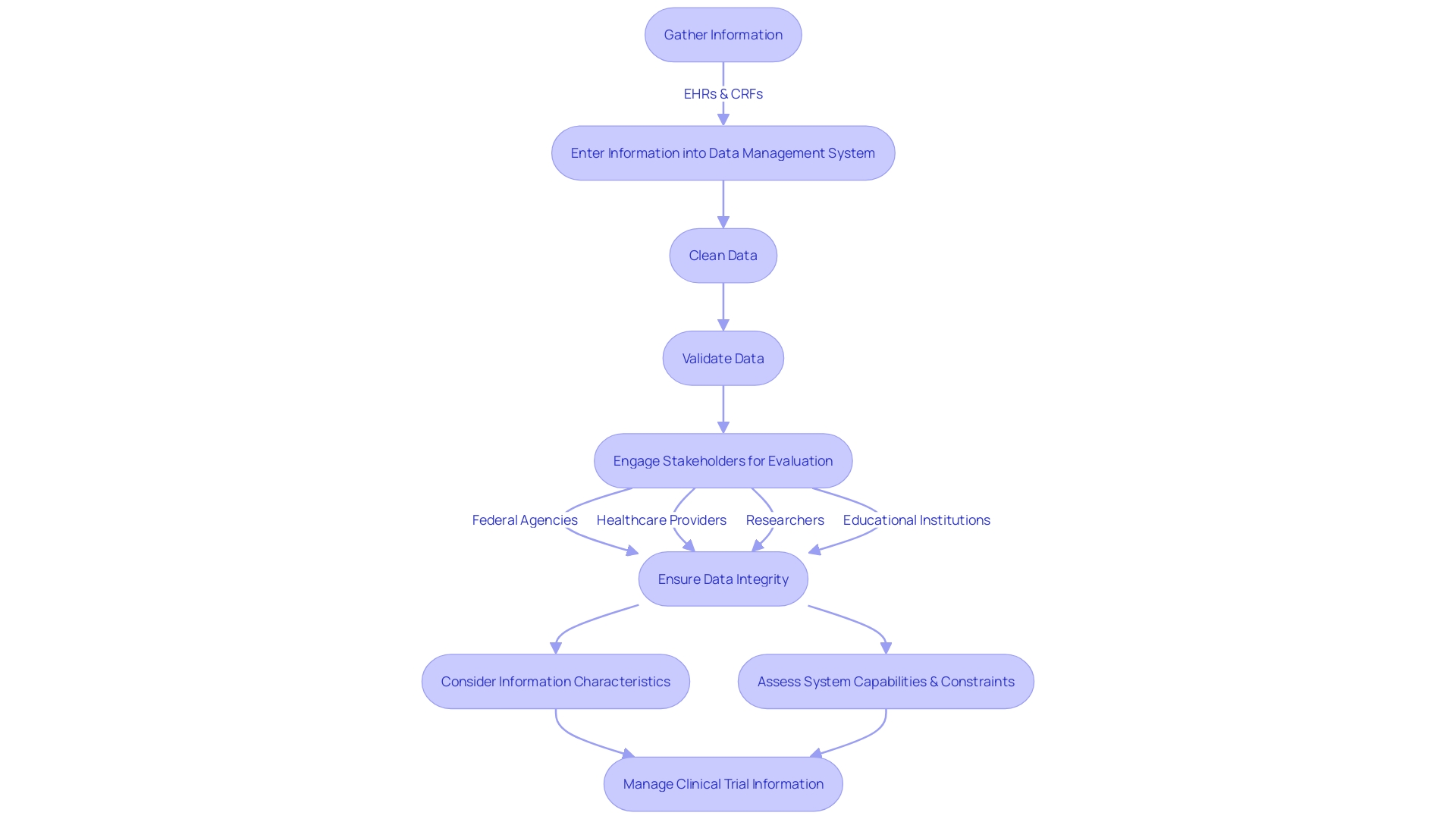
Thorough clinical information management is a crucial element of performing successful clinical experiments. It includes a sequence of important stages, specifically designed to maintain the integrity of trial information and safeguard patient safety. The procedure starts with careful information gathering, which integrates information from various sources such as electronic health records (EHRs) and case report forms (CRFs). Following collection, information is systematically entered into a specialized clinical data management system. This stage is crucial, as it establishes the groundwork for the subsequent cleaning phase. In this case, the information undergoes thorough scrutiny to detect and fix any inconsistencies or errors, guaranteeing the reliability of the dataset. The validation phase further strengthens this by thoroughly verifying accuracy and completeness. After successful validation, the database is locked, safeguarding the information against any additional modifications.
This organized method for handling information is not just essential for the safety of trial participants but also for the accuracy of outcomes, which could impact the well-being of numerous additional patients. Considering the Who's estimation of two million distinct medical devices available for use by a large population, it becomes apparent why careful control of clinical study information is essential.
Furthermore, the intricacy and amount of healthcare information frequently require subcontracting responsibilities, such as entering information, to external suppliers. This strategic move can enhance efficiency and accuracy while alleviating the burden on medical staff. It is particularly important in situations where manual information input is necessary due to the sensitive nature of medical records, as is the scenario with electronic medical records (EMRs) that require continuous updating.
In the realm of clinical information management, stakeholder engagement is crucial. Involving interested parties early on helps define the evaluation's context and objectives. Potential stakeholders might include federal agencies, healthcare providers, researchers, and educational institutions, all of whom have a vested interest in the information's application. The assessment of information should take into account its characteristics, such as the origin, participating institutions, and the time frame included, in addition to an evaluation of the information system's capabilities and constraints.
The importance of this strategic, multi-phase approach is underscored by innovative initiatives like Fresenius Medical Care's project, which leverages artificial intelligence to improve treatment protocols in dialysis patients. This project demonstrates the power of robust management of clinical information to offer significant insights and enhance the quality of life for patients on a global scale.
To promote openness and replicability in medical experiments, efforts have been made to enable wider availability of unprocessed experimental information. Nevertheless, the execution of such data-sharing necessities continues to be a field for expansion, with numerous experiments' comprehensive protocols and statistical analysis plans still not easily accessible.
To conclude, the life cycle of handling medical information is a vital part of the wider trial procedure, involving various parties and demanding strict compliance with optimal procedures to guarantee the gathering, processing, and evaluation of information is carried out with the highest precision and honesty.
Clinical Data Management Tools and Systems
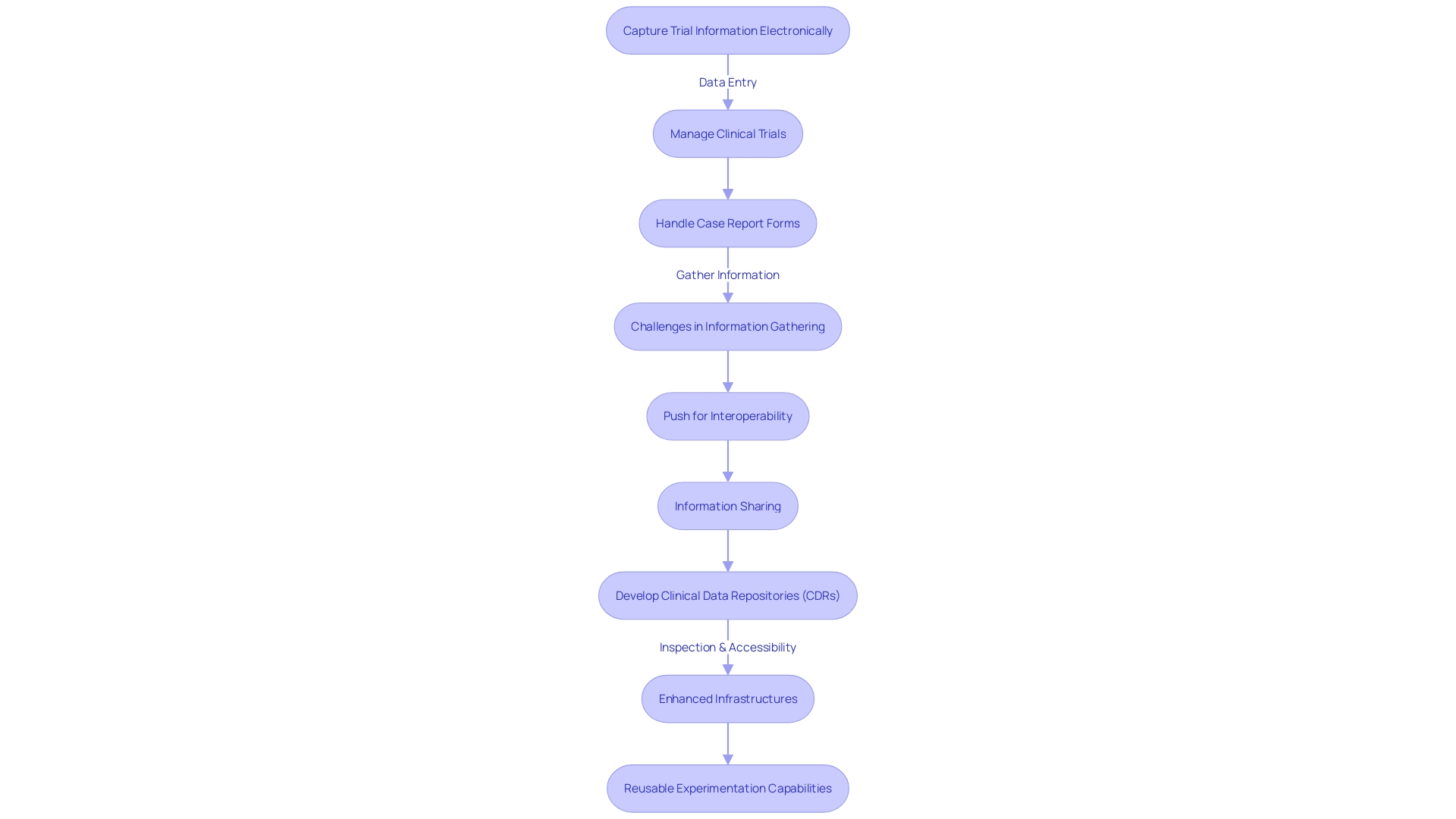
With the healthcare industry evolving rapidly, the handling of health information has become a central focus, especially considering the wealth of health information generated daily. To effectively navigate and derive value from this information, the industry is increasingly dependent on tools like Electronic Data Capture (EDC), Clinical Trial Management Systems (CTMS), and Electronic Case Report Form (eCRF) systems. These tools are not only aiding in the electronic gathering and organization of experimental information but are also simplifying processes and improving effectiveness overall.
EDC systems play a crucial role in capturing trial information electronically, guaranteeing the immediate accessibility and precision of this information. CTMS systems contribute by offering comprehensive tools for managing all aspects of clinical trials, simplifying complex project management tasks. Meanwhile, eCRF systems support the electronic creation, handling, and submission of case report forms, thereby reducing the manual effort involved in processing information.
Despite these advancements, challenges persist. Information gathering frequently stays limited within enclosed systems, distinct from current electronic health records (EHRs) or real-world information sources. This can lead to a duplication of efforts and missed opportunities to utilize existing information. Clinical trial information might capture only a transient snapshot of a participant's health, overlooking critical contextual information like social determinants of health, which are essential for understanding engagement, adherence, and retention in trials.
The industry is also witnessing a push for greater interoperability and information sharing, driven in part by legislative measures like the Health Information Technology for Economic and Clinical Health (HITECH) Act. The objective is to build a post-Electronic Health Record (EHR) era where information is not only digital but also universally accessible and valuable. Clinicians, however, still encounter the challenge of finding clinically relevant information within the extensive amount of information, often concealed in unstructured free-text notes within legacy EHR systems.
In response, the development of Clinical Data Repositories (CDRs) has been gaining traction. CDRs are patient-centered databases that offer real-time, readily accessible, and clinically focused information. They offer a longitudinal view of a patient's medical history, reduce redundant testing, and support risk modeling with intelligent algorithms. Information typically found in CDRs ranges from demographics to detailed medical records, facilitating a more holistic approach to patient care.
As we progress, the goal is evident: to enhance current infrastructures and establish a future with reusable experimentation capabilities. This will require not just embracing new technologies but also ensuring that these systems are designed to meet the stringent needs of managing clinical information - safeguarding participant safety and generating accurate, reliable information that can support regulatory submissions and ultimately enhance patient care.
Key Features of Electronic Clinical Data Management Systems
Electronic clinical systems (CDMS) are crucial in guaranteeing that clinical investigations are carried out with the utmost standards of precision, safety, and efficiency. These systems provide a range of capabilities essential for the careful handling of experiment information. Data entry forms, for instance, provide a structured and efficient interface for the input and organization of information, which is crucial considering that the World Health Organization approximates two million distinct categories of medical devices are available, each potentially utilized by millions of patients.
The significance of addressing inconsistencies in information cannot be emphasized enough, and query management systems are crucial in this procedure, protecting the accuracy of outcomes. Validation checks further strengthen this by ensuring the accuracy and completeness of information, crucial aspects as outlined by Article 62 of the European Union Medical Device Regulation (EU MDR) which emphasizes that clinical investigations must protect the rights, safety, dignity, and well-being of subjects, and produce scientifically valid, reliable, and robust information.
Data cleaning tools are another key feature of CDMS, designed to identify and rectify any errors, thereby preventing the propagation of inaccuracies that could compromise the quality of trial results. With the growing dependence on information-driven decision-making in healthcare, as demonstrated by the implementation of solutions like PRISMA by Bright Future Pediatrics for enhanced patient care, the importance of clean and precise information cannot be underestimated.
To bring information to life, reporting capabilities in CDMS enable the generation of detailed reports and summaries, facilitating a comprehensive analysis and review process. This feature aligns with the industry's shift towards more information-centric approaches, such as employing AI and data innovation to improve patient care outcomes—a subject of significant interest in recent discussions and webinars.
By applying the knowledge gained from different real-life examples, such as the digital modernization of Shield Medical Group to address 'care gaps' and the enhancement of the information system by Universal Health Services, it is evident that a strong CDMS is not just a tool but an essential requirement for the precise and regulatory handling of trial data. By implementing efficient information control, organizations can guarantee that their trials not only adhere to regulations but also add to the shared medical knowledge and progress of patient care.
Benefits of Using Electronic Clinical Data Management Systems
Electronic clinical information systems (CDMS) are increasingly becoming an essential component of modern clinical research, offering a multitude of advantages. These sophisticated systems are crucial in enhancing information precision and minimizing errors during information entry, thereby bolstering the integrity of the data. By simplifying the management process, they not only enable real-time access and monitoring but also support remote entry which is becoming more common.
The integration of CDMS in clinical research ensures that information is securely stored and readily accessible when needed. This ease of access is particularly beneficial for healthcare professionals who rely on swift information retrieval to inform decision-making processes. Furthermore, embracing electronic systems aids healthcare organizations in adhering to stringent regulatory standards, providing a robust framework for information traceability and audit trails.
In the context of healthcare quality improvement, such as the HEDIS® framework, transitioning to digital solutions has been shown to resolve inefficiencies like the 'gaps in care' issue faced by Shield Medical Group. By moving away from paper-based systems to a digital platform, they were able to consolidate patient and provider information, leading to enhanced efficiency in care delivery.
Additionally, the digital transformation of healthcare information is demonstrated by the transition from electronic medical records (EMRs) to electronic health records (EHRs). An EHR offers a comprehensive digital profile of a patient's health journey, updated in real-time with all pertinent health information, which is a step beyond the scope of EMRs that focus on a single healthcare setting.
The significance of effective management of factual medical information cannot be emphasized enough, as demonstrated by the European Union Medical Device Regulation, which highlights the safeguarding of trial participants and the production of accurate, dependable, and strong medical evidence. In the field of regulatory submissions, the evidence gathered from medical trials serves as the foundation that demonstrates the safety and effectiveness of medical devices.
Finally, progress in artificial intelligence (AI) in the domain of precision oncology and early cancer detection emphasize the crucial function of high-quality medical information. AI applications require precise and current information to guarantee the dependability of outcomes in patient care and promote the ongoing enhancement.
In general, the adoption of electronic CDMS is a priceless resource for medical research, guaranteeing that the complexities of information handling are dealt with extreme accuracy and effectiveness, thus promoting the excellence and results of patient care.
Best Practices for Clinical Data Management
Best practices in clinical information management are more than just routine procedures; they're a set of strategic guidelines that uphold the integrity and reliability of clinical trial data. It is crucial to establish standardized information collection methods that can handle the vast array of confidential patient information, medical histories, lab results, and insurance details. This is supported by the need for rigorous information validation checks and ongoing quality assessments, as highlighted in the analysis provided by Xtelligent Healthcare Media’s research division. Moreover, ensuring thorough documentation and strict compliance with regulatory guidelines, such as the European Union Medical Device Regulation (EU MDR), safeguards participants' rights and guarantees strong clinical information.
Ensuring that all team members are proficient in the latest digital healthcare solutions and compliant practices is a crucial part of this framework, which focuses on training personnel. This is echoed in the insights shared at WEDI’s Spring Conference, where the transformative role of AI in health information was discussed, emphasizing the need for a knowledgeable team to leverage such advanced technologies effectively.
Regular updates and maintenance of information systems in the medical field are essential. Shield Medical Group's transition from paper-based processes to a digital solution exemplifies this, highlighting the operational efficiencies gained by adopting IT solutions tailored for healthcare. Real-world use cases, such as the machine learning tool predicting spine surgery outcomes, demonstrate how cutting-edge technology, when properly managed, can lead to significant improvements in care quality and outcomes.
Overall, by integrating these best practices, healthcare institutions can navigate the challenges of medical data management, ensuring the generation of scientifically valid, reliable, and robust clinical data that forms the backbone of regulatory submissions and ultimately safeguards patient safety and wellbeing.
Conclusion
In conclusion, comprehensive clinical data management is crucial for the integrity of clinical trials and the assessment of medical device safety and efficacy. With over two million types of medical devices globally, rigorous data management is pivotal.
Roles such as data managers, database programmers, biostatisticians, and quality control personnel play a vital role in maintaining data integrity and participant safety. The field of informatics also contributes to optimizing patient care.
Phases of clinical data management, including data collection, cleaning, and validation, are essential for maintaining data integrity and patient safety. Electronic data management systems and tools streamline data collection and management, improving efficiency and accuracy.
Key features of electronic clinical data management systems, such as data entry forms and query management systems, ensure data accuracy and integrity. These systems offer reporting capabilities for comprehensive analysis.
Benefits of using electronic clinical data management systems include enhanced data precision, real-time access, remote data entry, and adherence to regulatory standards. These systems support efficient information retrieval and compliance.
Best practices in clinical data management involve standardized data collection methods, validation checks, comprehensive documentation, and adherence to regulatory guidelines. Training personnel and regular updates of data management systems are crucial.
In summary, comprehensive clinical data management is crucial for the integrity of clinical trials and medical device assessment. By implementing best practices and utilizing electronic data management systems, healthcare organizations can ensure data accuracy, integrity, and patient safety, ultimately advancing medical knowledge and improving patient care.




