Introduction
The process of obtaining FDA clearance for medical devices in the United States is a crucial step for manufacturers. The 510(k) premarket notification process, designed for devices not subject to the more rigorous Pre-Market Approval (PMA) or De Novo classification, requires demonstrating substantial equivalence to a legally marketed predicate device. This article provides a comprehensive overview of the 510(k) process, including the types of submissions, key components of a submission, the timeline for review and approval, and tips for a successful submission.
By understanding the intricacies of the 510(k) process and adhering to FDA guidelines, manufacturers can navigate the regulatory landscape and ensure the safety and effectiveness of their medical devices.
Understanding the 510(k) Process
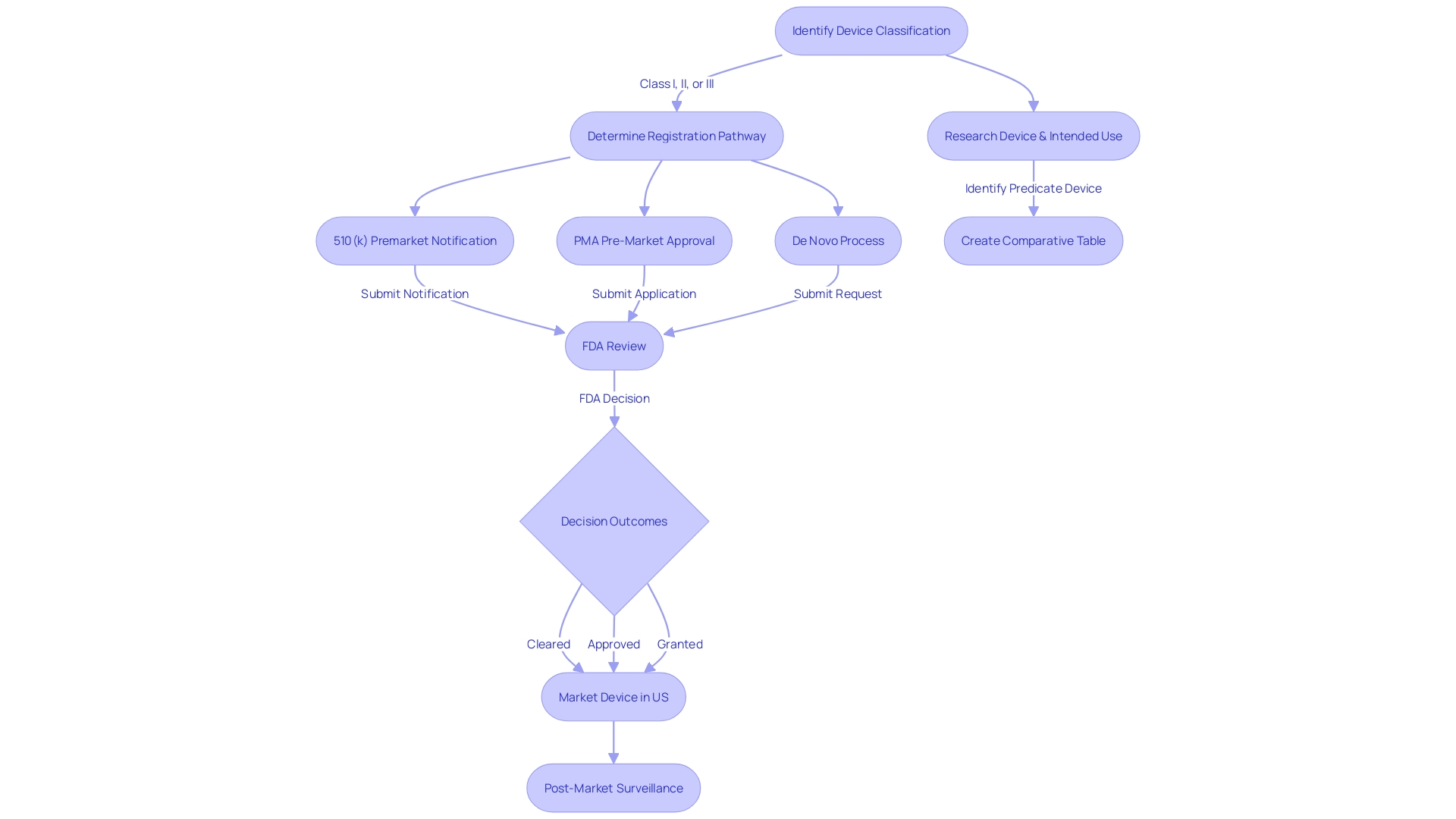
Successfully navigating the FDA's 510(k) premarket notification process is crucial for medical product manufacturers aiming to market their products in the United States. This regulatory pathway, designed for products that are not subject to the more rigorous Pre-Market Approval (PMA) or De Novo classification, relies on demonstrating that a new item is substantially equivalent to a legally marketed reference product. Substantial equivalence means the new product has the same intended use and similar technological characteristics or that any differences do not raise new safety and effectiveness questions.
To start, manufacturers must have a comprehensive understanding of the equipment, its intended purpose, and the potential user base, which may consist of clinicians, physicians, dentists, and patients. It is crucial to analyze all accessible materials, such as research literature, clinical studies, and competitor information, to develop a comprehensive understanding of the object's context in the market. Identifying a suitable predicate involves a thorough review of resources like the FDA’s 510(k) database, which contains Summaries of Safety and Effectiveness (SSEs).
Understanding the distinctions between FDA terms such as registered, cleared, approved, and granted is also fundamental. These classifications are significant, as they represent differing levels of regulatory clearance and patient risk. The FDA categorizes medical equipment into three groups according to their level of risk, and this categorization determines the suitable registration pathway.
The 510(k) process does not always require clinical trials, which was a point of critique in the 2018 documentary 'The Bleeding Edge.' This documentary highlighted instances where devices cleared through the 510(k) process led to patient injuries. As public scrutiny and legislative policies evolve, the FDA continues to update its guidance, including the recent publication of standards for direct-to-consumer prescription drug advertisements, emphasizing the need for clarity, conciseness, and neutrality in the presentation of major side effects.
In the end, the preparation of the 510(k) involves a comprehensive comparative analysis, ensuring that all necessary information is included. This information may encompass technical data, labeling, and any necessary clinical or scientific data. The manufacturer must ensure that the document is complete and that any comments made during the process do not contain confidential information. The FDA's commitment to public health and safety underscores the importance of adhering to these rigorous standards throughout the 510(k) clearance process.
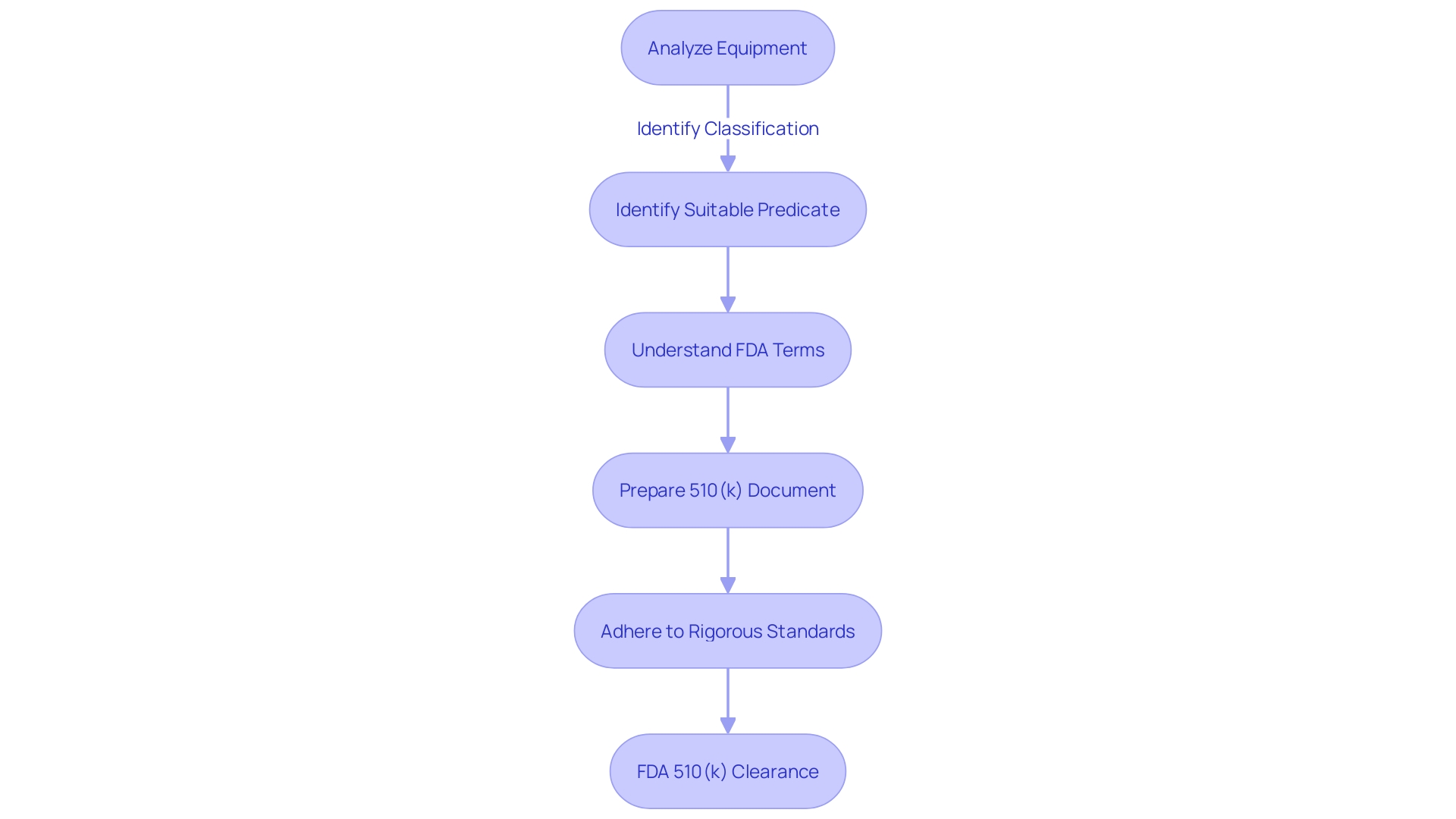
Types of 510(k) Submissions
Comprehending the intricacies of 510(k) applications is vital for medical tool producers pursuing FDA approval. A significant portion of the process of submitting involves showcasing 'Substantial Equivalence' to a predicate apparatus, which implies that the novel apparatus has the identical intended purpose and is equally safe and efficient as an already lawfully marketed apparatus, without raising fresh concerns of safety or effectiveness. There are three main paths to achieve this: Traditional, Special, and Abbreviated 510(k) submissions.
The Traditional 510(k) route is the most commonly used path. It necessitates an extensive comparison with one or more predicate apparatus. Manufacturers should deeply engage with the subject product, understanding its use, users, and instructions, and ensure a thorough competitive landscape analysis. To support this, a comparative table showcasing the similarities and differences in technological characteristics with the predicate object should be constructed, leveraging research literature, clinical studies, and existing regulatory summaries.
For the Special 510(k) submission, it's applicable when a manufacturer makes modifications to its own FDA-cleared product. This pathway allows for a more streamlined review by the FDA, provided that the modifications do not affect the intended use or alter the fundamental scientific technology of the product.
Lastly, the Abbreviated 510(k) option leverages guidance documents, special controls, and recognized standards to demonstrate compliance. This can be particularly advantageous when there are clear FDA recommendations on the assessment of certain device types.
It is worth mentioning that most initial 510(k) applications encounter denial, and a considerable portion of these are attributed to problems concerning establishing Substantial Equivalence. This emphasizes the significance of a well-prepared document that closely follows FDA's requirements. The FDA continually updates its processes and guidelines, as seen in recent meetings focused on enhancing systematic post-market evaluations. Staying informed on these updates is essential for successful applications and ensuring public health protection.
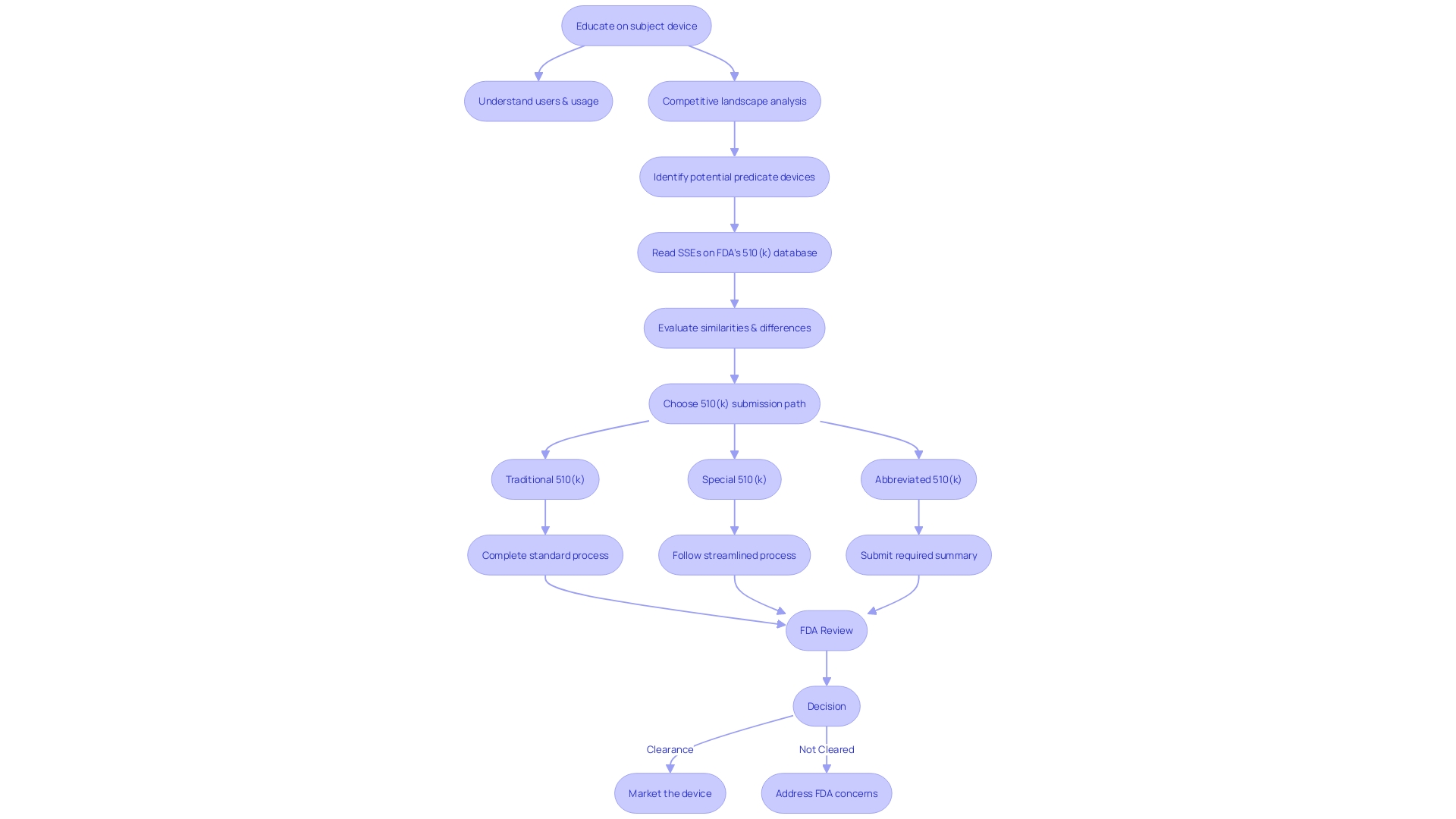
Key Components of a 510(k) Submission
Compiling a 510(k) is a meticulous process that involves detailed documentation to demonstrate a medical device's safety and effectiveness. The document must include a Medical Device User Fee Cover Sheet, which outlines the payment of fees associated with the review process by the FDA. A Premarket Review Cover Sheet is also necessary, serving as a formal introduction and summary of the contents.
A Truthful and Accuracy Statement, certified by the submitter, attests to the veracity of the information provided within the submission. For Class III items, a summary of the object's safety and effectiveness, along with a certification, is required. Financial Certification or Disclosure Statements are also included to disclose any potential financial conflicts of interest.
To verify that the equipment complies with applicable standards, Declarations of Conformity and Summary Reports are provided. A comprehensive Description clarifies the purpose, construction, and function of the apparatus, providing understanding into its design and usage.
Proposed Labeling must be included to detail instructions for use, safety warnings, and other pertinent information for end-users. Information on Sterilization and Shelf Life is crucial for ensuring the equipment's safety and longevity. Lastly, Biocompatibility data must be presented to confirm that the equipment is compatible with human tissue and does not pose any undue risks.
Every element of the 510(k) submission acts as a vital piece of evidence in the FDA's evaluation process, guaranteeing that medical instruments adhere to the stringent standards necessary to protect public health.
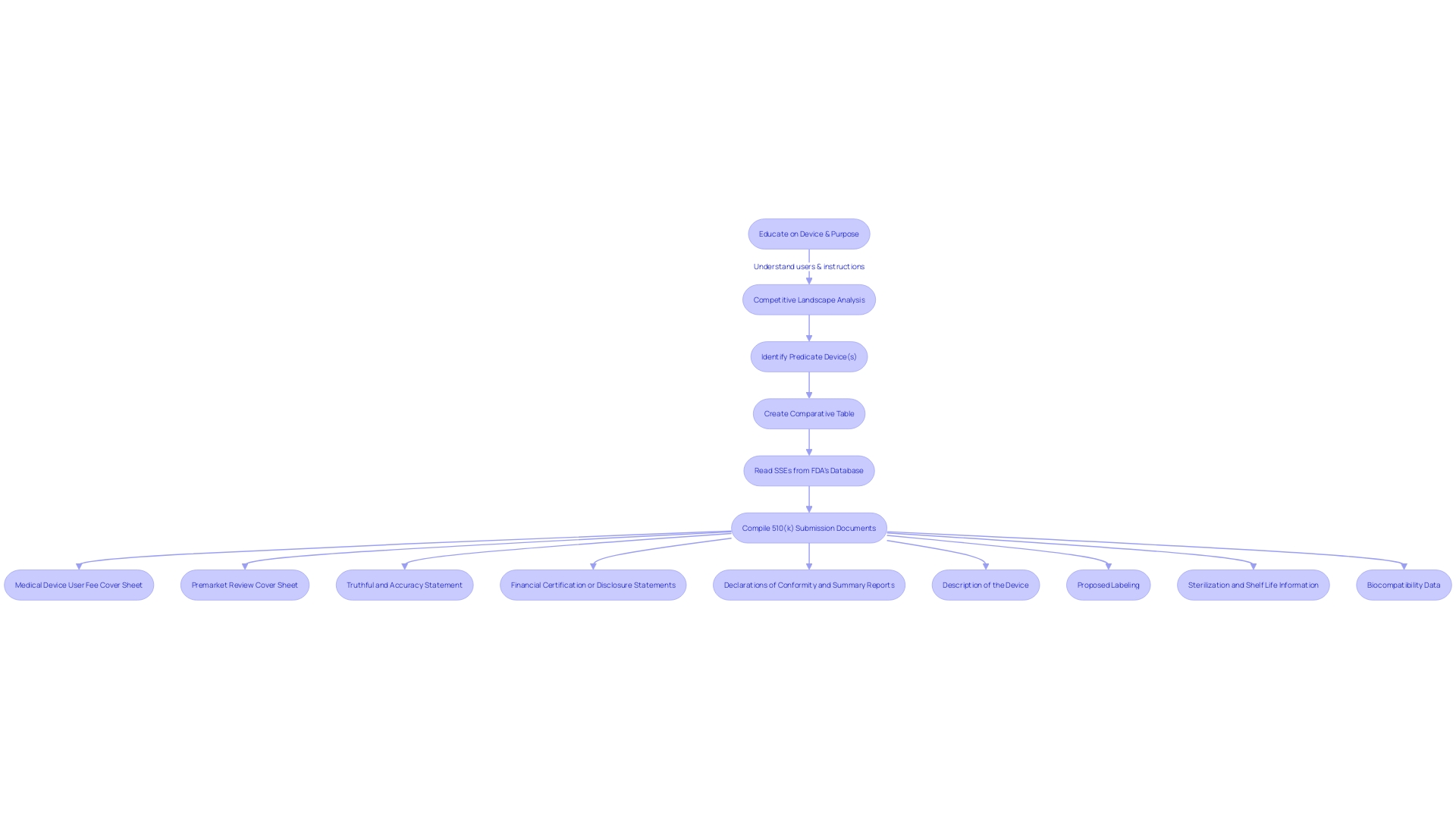
Submission and Review Process
The process of obtaining FDA clearance for a medical instrument through the 510(k) pathway involves a meticulous review to ensure the instrument complies with regulatory standards. At first, it is crucial to gain a thorough comprehension of the object in question, its users, and its applications, which could encompass a range of individuals from clinicians and patients to dentists and physicians. This comprehension should cover the instructions of the apparatus, encompassing any alerts and cautions connected to its utilization.
To identify a suitable alternative, one with the same intended use and similar technological characteristics, a thorough examination of the competitive landscape is necessary. This includes evaluating a range of materials like research literature, clinical studies, and marketing materials from analogous products. Creating a comparative table to document similarities and differences between the device under review and the potential predicate is an essential step. Moreover, consulting the FDA’s 510(k) database and studying the Summaries of Safety and Effectiveness (SSEs) can provide valuable insights into the predicates' performance and safety profiles.
During the process of providing comments and documentation to the Health and Human Services Department, the public nature of the procedure is emphasized. All documents are made public post-review, emphasizing the significance of excluding confidential information, such as medical data or confidential business information, from these records. For those who wish to provide confidential information, it is advised to adhere to the detailed instructions for written submissions to ensure the information is protected.
In summary, the FDA's 510(k) submission and review process is not just a regulatory hurdle but a crucial phase that safeguards public health by scrutinizing the safety and efficacy of medical equipment. The process ensures that once an instrument is brought to market, it has undergone rigorous evaluation and is deemed safe for use by both healthcare professionals and patients.
Timeline for 510(k) Review and Approval
The process of obtaining FDA 510(k) clearance is intricate and demands a thorough understanding of both the product in question and the regulatory landscape. A typical 510(k) submission review involves several stages, with the duration of each hinging on the complexity of the equipment and the thoroughness of the application submitted.
To navigate this process effectively, it is crucial to start by gaining an in-depth understanding of the equipment's users, its applications, and any associated warnings. In collaboration with Marketing teams, it is equally important to analyze the competitive environment, identifying and comparing potential predicate products that share similar intended uses and technological characteristics.
Grasping the idea of substantial equivalence is crucial, as a substantial proportion of initial 510(k) filings fall short due to problems in demonstrating this similarity. FDA defines substantial equivalence as a device having the same intended use as a predicate device, and either the same technological characteristics or sufficient data to prove it is as safe and effective as the predicate.
The 510(k) review process also requires attention to detail when creating the documents for review. Submitters should adhere to the FDA's formatting guidelines and thoroughly review their documentation to ensure no confidential information is inadvertently disclosed.
Statistics emphasize the crucial importance of adequately addressing substantial equivalence, with 75% of 510(k) filings being rejected on the initial try, 85% of which are due to concerns regarding substantial equivalence. Thus, conducting a comparative analysis and examining the Summaries of Safety and Effectiveness discovered in the FDA’s 510(k) database becomes a vital component of the strategy.
In general, the timeframe for 510(k) approval can vary, but by implementing a strategic method based on a thorough comprehension of the product, the regulatory structure, and a well-prepared application, the probability of a successful evaluation process is greatly improved.
Common Mistakes and Tips for Successful Submission
Developing a prosperous FDA 510(k) application is a challenging endeavor, necessitating a thorough comprehension of the medical equipment, its intended purpose, and the market environment. To evade typical traps and guarantee your submission is well-received by the FDA, it is crucial to extensively investigate and comprehend the equipment's users, spanning from clinicians and physicians to patients, and to scrutinize the instructions for use with a fine-tooth comb for any warnings and cautions.
The competitive landscape should also be examined with a detailed eye, identifying potential objects that share the intended use and similar technological characteristics to your own. This can be achieved by analyzing research literature, clinical studies, and marketing materials such as brochures and sell-sheets. Generating a comparative table can be especially effective in showcasing the similarities and differences between your product and existing ones on the market.
Moreover, acquainting yourself with the Summaries of Safety and Effectiveness (SSEs) accessible on the FDA's 510(k) database will offer valuable insights into how the FDA has assessed precursor devices, which can guide your own approach for presenting.
A comprehensive preparation approach is essential, and it's important to utilize all available resources, including the FDA's guidance documents and tools for submitting. For instance, the documentary 'The Bleeding Edge' highlights the importance of understanding the FDA's 510(k) clearance process and the concept of substantial equivalence. By ensuring that your equipment matches a predicate in terms of intended use and technological characteristics, or by providing robust scientific data to support different technological characteristics, you can meet the FDA's criteria for substantial equivalence.
Ultimately, the key to a smooth 510(k) submission process is the meticulous gathering and presentation of all pertinent information, ensuring it adheres to the FDA's expectations. By doing so, you increase the likelihood of a favorable review and clearance, allowing your medical device to reach the market more swiftly.
Conclusion
In conclusion, navigating the FDA's 510(k) premarket notification process is crucial for medical device manufacturers in the US. Understanding the different types of submissions and key components of a 510(k) submission is essential for a successful clearance. Adhering to FDA guidelines and staying informed on updates ensures the safety and effectiveness of medical devices.
The timeline for 510(k) review and approval varies, but with a strategic approach and a well-prepared submission, manufacturers can enhance their chances of a favorable outcome. Thorough research, analyzing the competitive landscape, and leveraging available resources like FDA guidance documents are vital for a smooth submission process.
By avoiding common mistakes and following these tips, manufacturers can increase their chances of a favorable review and clearance, allowing their medical devices to reach the market efficiently. The 510(k) process plays a vital role in ensuring the safety and efficacy of medical devices, and adherence to FDA guidelines is crucial for navigating the regulatory landscape with confidence.




