Introduction
The FDA 510(k) submission is a crucial gateway for medical device manufacturers to bring their products to market. It establishes that a device is substantially equivalent to an existing device already on the market, prioritizing patient safety while avoiding extensive clinical trials. To achieve this, an in-depth understanding of the device, its intended users, and the competitive landscape is vital.
The FDA classifies medical devices into three categories based on risk, and selecting the correct classification is essential for determining the appropriate registration pathway. Confidentiality is crucial when submitting comments or documentation to the FDA, and careful selection of predicate devices and thorough research are necessary to demonstrate substantial equivalence. The 510(k) submission process is a comprehensive and strategic approach that ensures safety and efficacy for public health.
What is an FDA 510(k) Submission?
The FDA 510(k) submission serves as a pivotal gateway for medical equipment manufacturers to introduce their products into the market, asserting that their apparatus is substantially equivalent to an existing instrument legally on the market, known as a predicate device. The pathway prioritizes patient safety by requiring a thorough comparison with a reference, thereby allowing new products to be marketed without necessitating extensive clinical trials.
A comprehensive understanding of the equipment in question, its intended users, such as clinicians and patients, and the accompanying instructions for use, complete with warnings and cautions, is of utmost importance. Working together with marketing teams gives perspective on the competitive environment, emphasizing the presence of rival products. Such collaboration is crucial in identifying potential predicate tools sharing similar intended uses and technological characteristics, which can be facilitated by reviewing research literature, clinical studies, and marketing materials.
The creation of a comprehensive comparative table is a strategic step in this process, which should include reading and evaluating the Summaries of Safety and Effectiveness Data (SSEs) available on the FDA's 510(k) database. This analysis ensures a comprehensive comprehension of both the similarities and distinctions between tools, which is crucial for building a compelling argument for substantial equivalence.
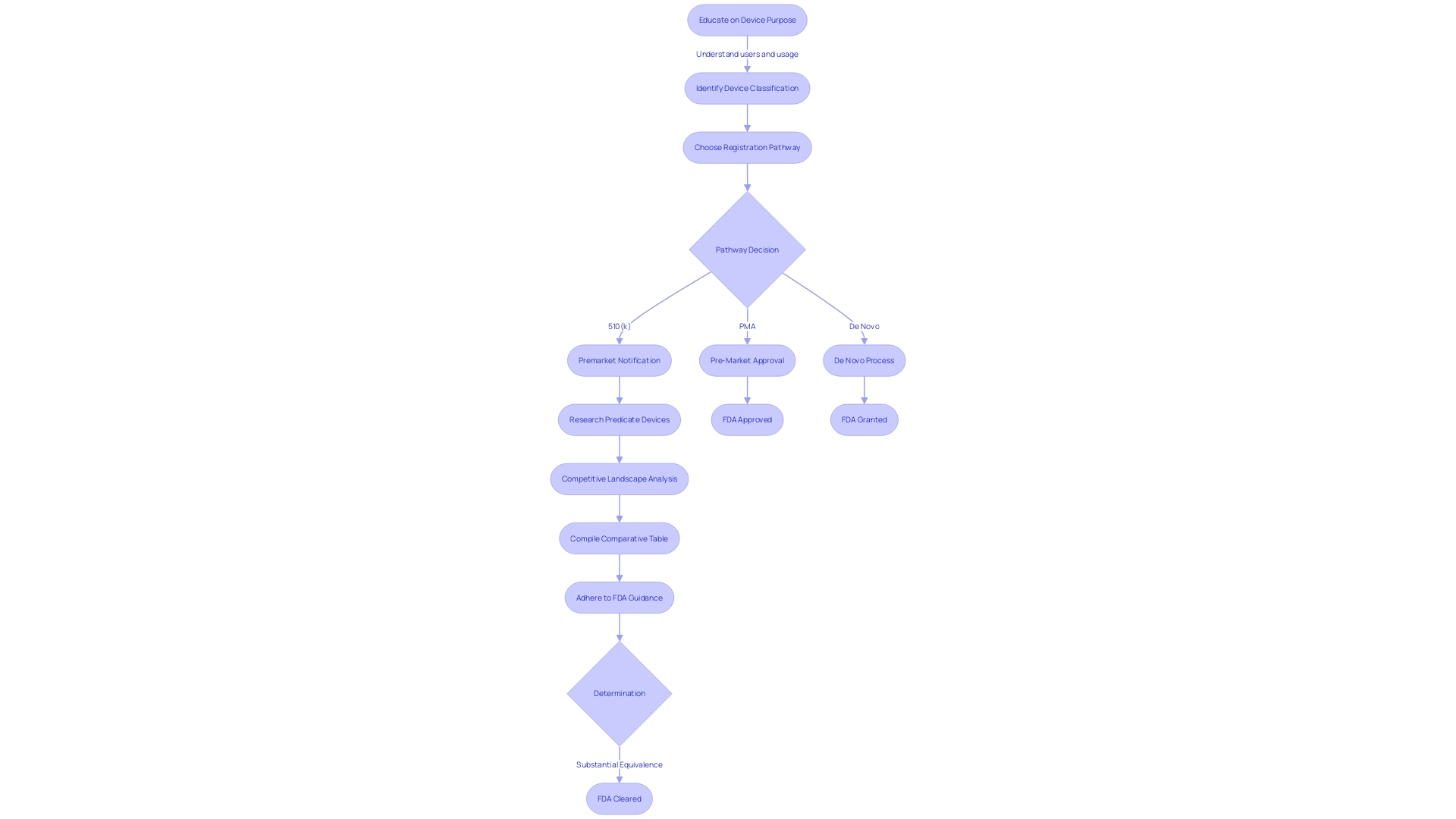
Furthermore, the FDA categorizes medical equipment into three classifications based on the risk they pose to patients. Identifying the correct classification is a critical step before selecting the appropriate registration pathway, whether it be the Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo process. The terminology used by the FDA—Registered, Cleared, Approved, and Granted—carries distinct meanings and implications, and it is crucial for regulatory professionals to comprehend these nuances.
Lastly, when submitting comments or documentation to the FDA, it is imperative to ensure that no confidential information is disclosed publicly unless intended. If confidentiality is desired, the document should follow the detailed written/paper process as specified by the FDA.
In summary, the 510(k) submission process is not merely a regulatory formality but a comprehensive, strategic approach that necessitates a deep dive into the equipment's technology, market positioning, and regulatory classification to ensure safety and efficacy for public health.
Medical Device Classes and 510(k) Submissions
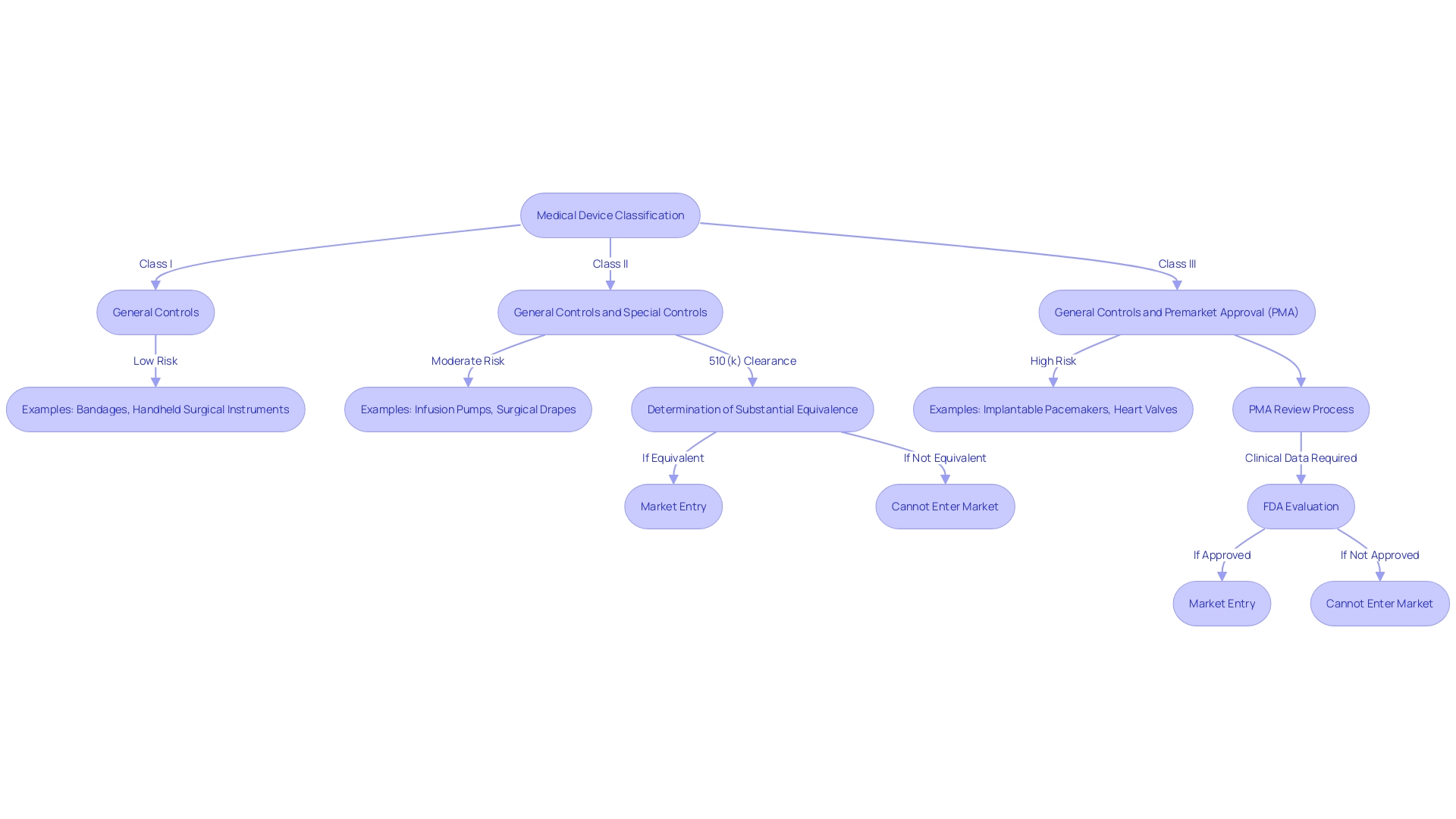
Medical instruments, crucial tools in healthcare, are strictly regulated to ensure patient safety. The U.S. Food and Drug Administration (FDA) categorizes these instruments into three classifications, each representing the degree of risk they pose and establishing the required regulatory controls. Class I products are considered low-risk and often require the least regulatory supervision. Examples include dental floss and elastic bandages. Class II products present moderate risk and typically necessitate more regulatory controls to ensure safety and effectiveness; these include powered wheelchairs and some pregnancy test kits. 'Class III products, like implantable pacemakers, are high-risk and typically necessitate the most rigorous regulatory scrutiny, including premarket approval (PMA).'.
The FDA's 510(k) clearance process allows Class II and certain Class I products to be marketed without requiring clinical trials if they are substantially equivalent to a predicate item already on the market. Nevertheless, this procedure has encountered disapproval, as brought to attention by the 2018 documentary 'The Bleeding Edge,' which uncovered instances of patient harm caused by instruments that were expedited through this route. The FDA continues to evolve its regulatory processes, as evidenced by recent updates to advertising regulations, ensuring the clear and neutral presentation of side effects in direct-to-consumer ads.
For those in the medical equipment industry, identifying a reference product is a critical step in the 510(k) submission. Comprehending the intended use, technological characteristics, and the competitive landscape of the instrument is crucial. This involves researching FDA's databases for Summaries of Safety and Effectiveness and creating comparative tables to support the claim of substantial equivalence.
In summary, the categorization of a medical apparatus by the FDA determines the regulatory prerequisites for its market entry. The 510(k) clearance process plays a crucial role for Class II and certain Class I instruments, with the objective of striking a balance between patient safety and innovation in the medical equipment sector.
Who Needs to Submit a 510(k)?
Knowing which medical equipment necessitates a 510(k) filing is crucial for guaranteeing adherence to FDA rules. Devices that are exempt from this requirement include those manufactured or altered solely for use in a specific practitioner's practice, such as by physicians or dentists, and those intended for research, teaching, or analysis without entering commercial distribution. Furthermore, pharmacies and retail establishments that provide or sell products directly to the end user, as well as carriers that transport items as part of their business, are also exempt.
Determining if a product necessitates a 510(k) filing entails evaluating its classification, which is established by the degree of risk it presents to patients. Devices are categorized into three classifications by the FDA, with Class I posing the least risk and Class III the most. The categorization of the tool determines the suitable registration pathway, which may involve a 510(k) application, Pre-Market Approval (PMA), or the De Novo procedure.
It is crucial to identify a lawfully marketed predecessor with the same intended use and similar technological characteristics when considering a 510(k) submission. The FDA's 510(k) database provides Summaries of Safety and Effectiveness (SSEs) that can be instrumental in evaluating the similarities and differences between products.
The significance of this evaluation process was emphasized in the documentary 'The Bleeding Edge', which exposed that certain equipment were expedited without the requirement for clinical trials, resulting in unfavorable patient outcomes. This underscores the significance of careful predicate selection and the analysis of long-term safety data.
The FDA has recently focused on modernizing the 510(k) process, including the development of best practices for selecting predicates. These efforts aim to improve the review process and enhance safety of the equipment, as indicated by the draft guidance document released in September 2023.
Medical instruments are an essential element of patient care, and their safety is of utmost importance. According to a study conducted in 2018, in the span of ten years, there were possibly over 1.7 million injuries and 83,000 deaths in the United States that could be attributed to medical equipment. These figures highlight the necessity for stringent regulatory oversight and active postmarket surveillance to safeguard public health.
The Concept of Substantial Equivalence and Predicate Devices
Grasping the notion of 'substantial equivalence' is crucial when maneuvering through the 510(k) submission process for medical apparatus. Substantial equivalence is determined by comparing a new medical instrument with one or more existing instruments, known as precedent instruments, which have been previously cleared by the FDA. The comparison focuses on intended use and technological characteristics, ensuring the new equipment is at least as safe and effective as its predicate.
When identifying a suitable predicate instrument, it is crucial to conduct thorough research, which includes analyzing the users, such as clinicians and patients, and understanding the instrument's instructions for use, warnings, and cautions. By examining competing products, marketing materials, research literature, and clinical studies, one can generate a comparative table that emphasizes the similarities and distinctions with potential reference devices.
The World Health Organization defines a medical tool broadly, encompassing items ranging from simple medical thermometers to complex diagnostic software and prosthetic implants. This underscores the importance of the FDA’s role in ensuring safety and effectiveness through regulatory oversight. The design and utility of medical instruments are critical components in the healthcare landscape, significantly contributing to patient care and quality of life.
Public commentary and feedback play a role in the regulatory process, with the FDA providing clear guidelines to ensure that any submitted comments do not disclose confidential information. For those submitting comments, it is crucial to adhere to these instructions to protect sensitive data.
In recent years, the FDA's 510(k) clearance process has faced scrutiny for its approach to expediting products based on substantial equivalence without requiring clinical trials in all cases. The assessment of Safety and Effectiveness Summaries (SSEs) from FDAâs 510(k) database is a valuable resource for comprehending how previous products have been evaluated, offering insight into the agency's decision-making process.
Components of a 510(k) Submission
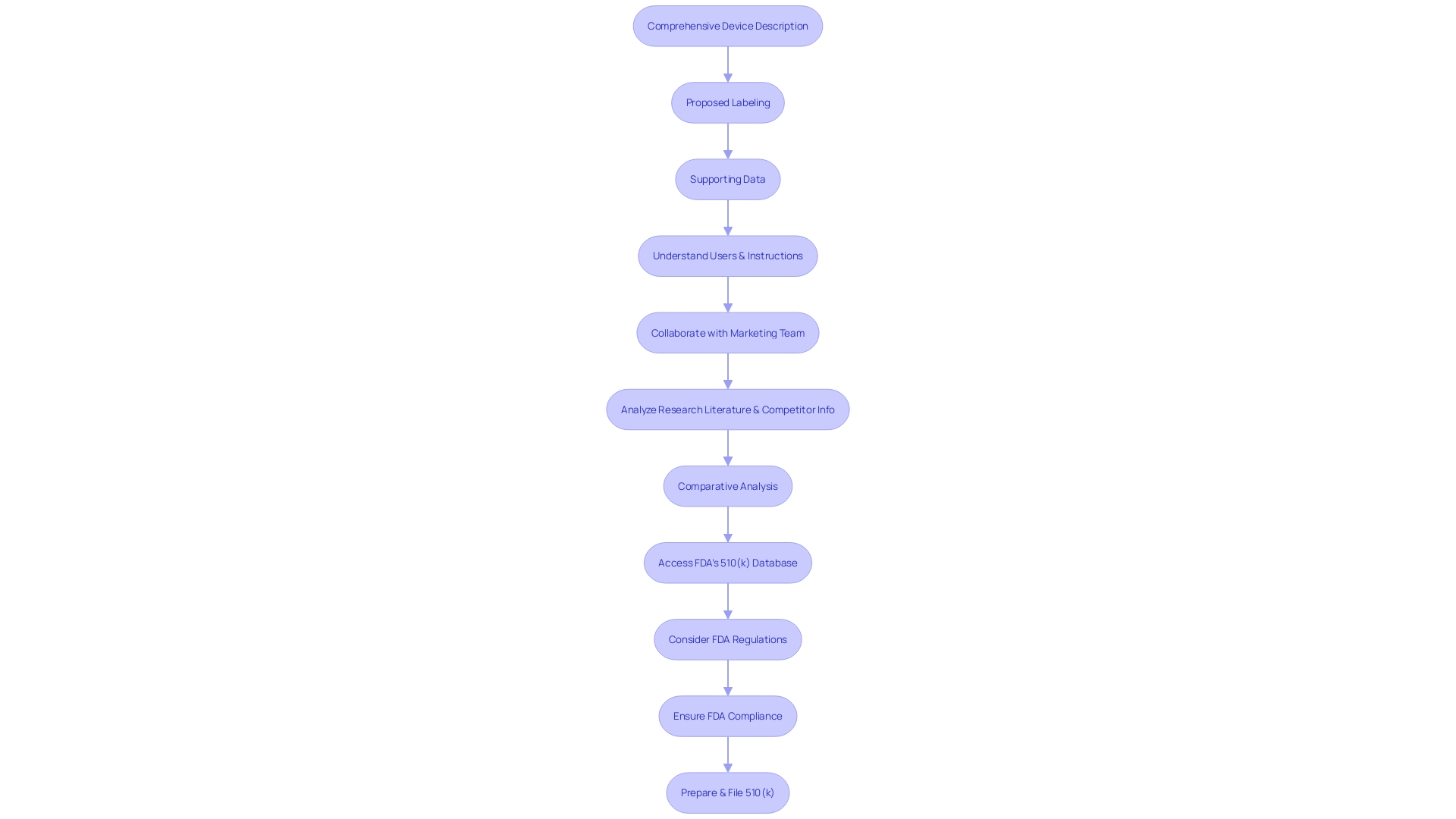
The intricacies of a 510(k) submission are pivotal for its success. Essential components to be carefully integrated include a comprehensive description of the apparatus, proposed labeling, and strong supporting data. This extensive collection is essential to comply with the rigorous guidelines established by the U.S. Food and Drug Administration (FDA), ensuring the safety and effectiveness of medical equipment.
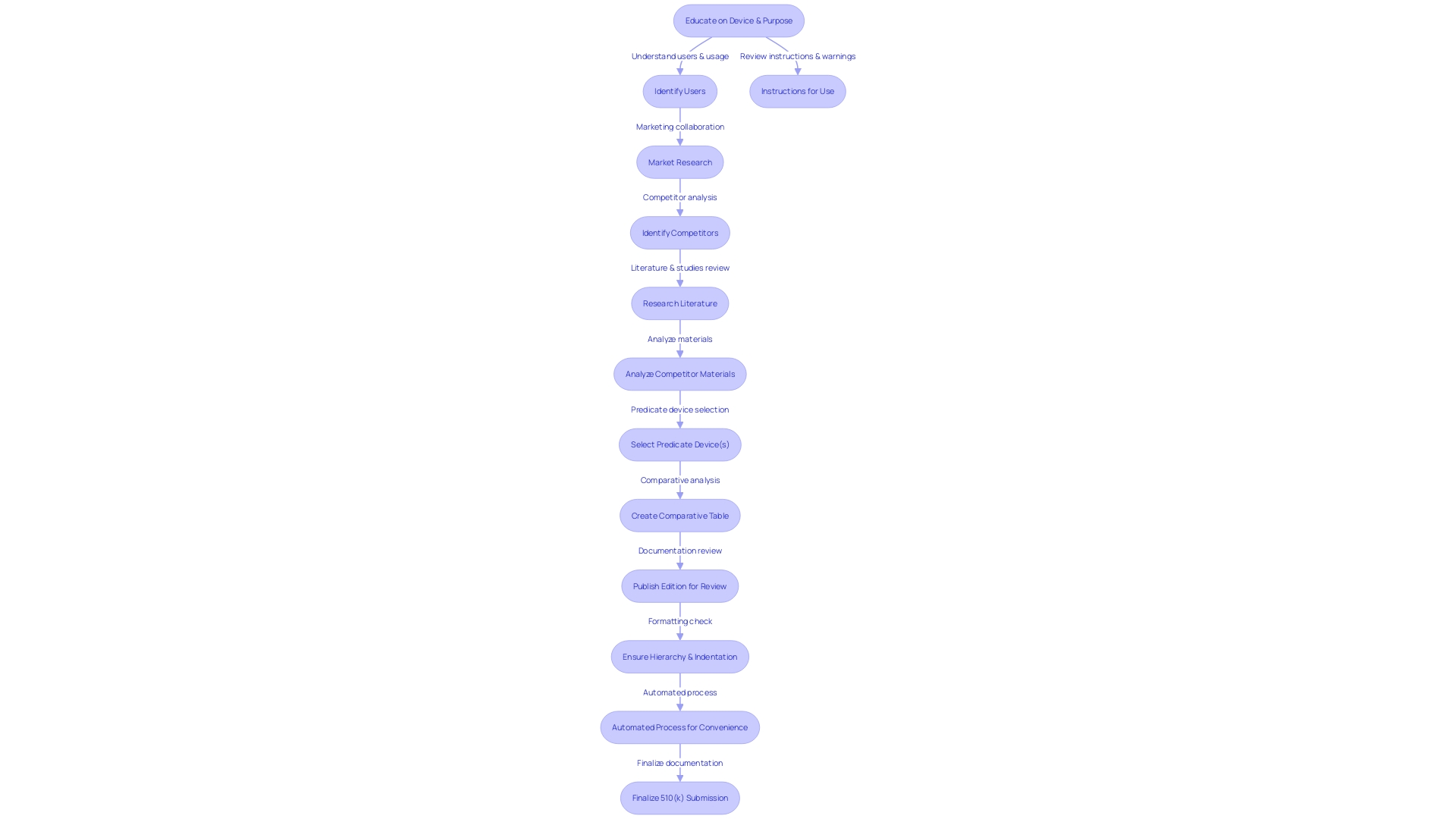
Understanding your medical equipment fully is crucial. This requires extensive understanding of the users of the apparatus, which can include clinicians, physicians, and patients, as well as comprehending the instructions for use, including warnings and cautions. Collaborating with your marketing team is beneficial to grasp the competitive environment thoroughly. Analyzing research literature, clinical studies, and competitor information will assist in identifying a suitable predicate apparatus with analogous intended use and technological characteristics. A comparative analysis is essential in this process.
The FDA's 510(k) database is a valuable resource, providing access to Summaries of Safety and Effectiveness (SSEs) that offer insights into the similarities and differences between your apparatus and its predicates. Furthermore, it is crucial to take into account the FDA's function in safeguarding public health through the regulation of various products, including medications, medical equipment, food supply, and cosmetics, which highlights the agency's thorough supervision and the necessity for strict adherence in applications.
A case in point where the lack of validation for aseptic processes and appropriate written procedures for environmental monitoring led to significant compliance issues, highlights the importance of ensuring all processes are FDA-compliant. This illustrates the need for a comprehensive and systematic approach to 510(k) preparation.
Moreover, during the autumn of 2022, a documentary named 'The Bleeding Edge' brought attention to the FDA's 510(k) clearance procedure, exposing that certain equipment were expedited without obligatory clinical trials, resulting in harm to patients. This emphasizes the significance of thorough preparation and filing to safeguard patient safety and maintain the integrity of the medical instrument market.
The 510(k) Submission Process
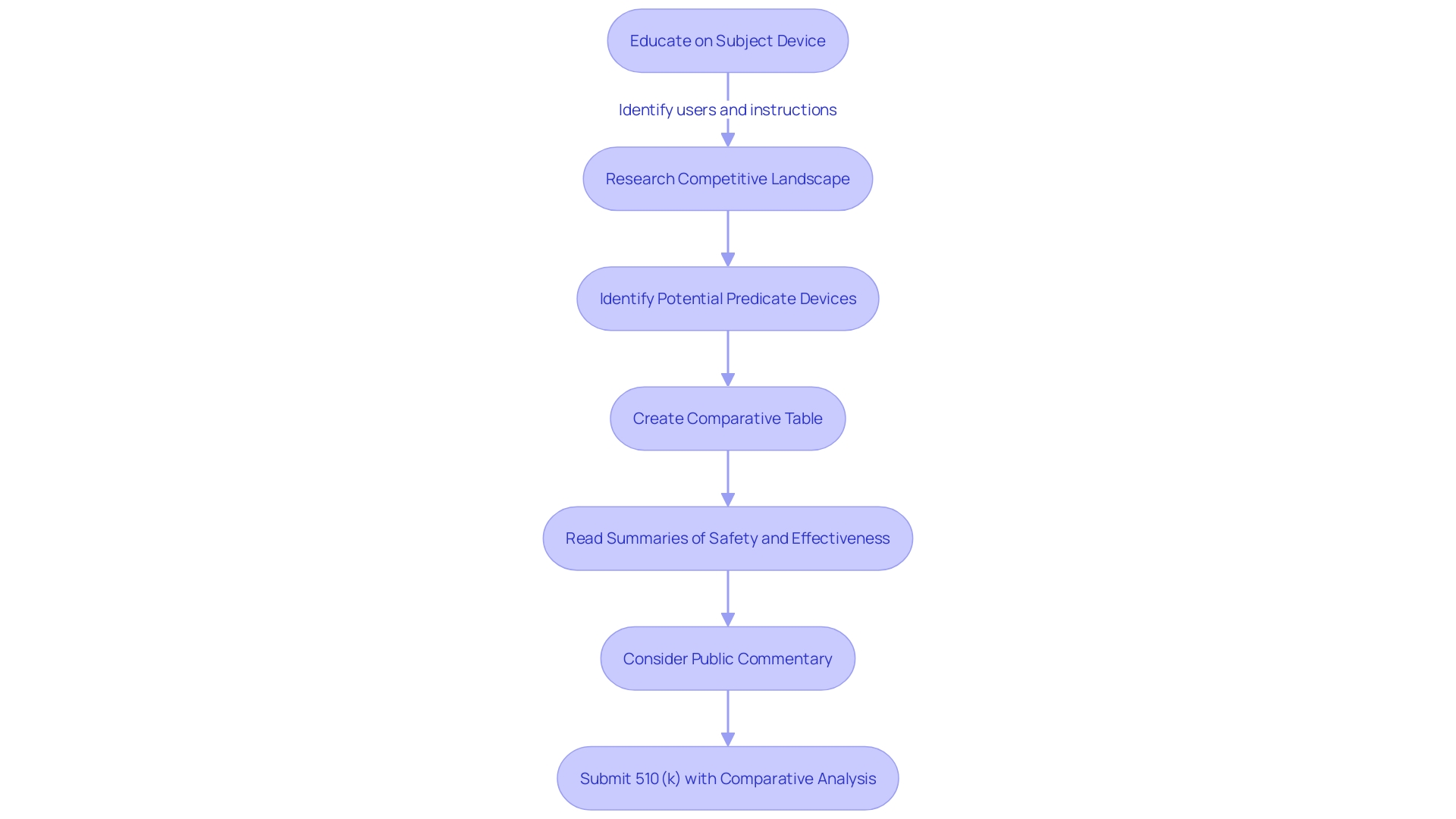
Navigating the FDA's 510(k) submission process is akin to assembling a complex puzzle, requiring a meticulous understanding of the medical instrument in question. This entails acquiring extensive understanding about the users of the apparatus, including clinicians, physicians, dentists, and patients, in addition to a thorough examination of the instructions for usage, encompassing all warnings and precautions.
A strategic approach involves working together with the marketing department to examine the competitive environment, with a specific emphasis on similar products already present in the market. Essential to this analysis is the examination of a wide array of resources including research publications, clinical studies, and marketing materials like websites, brochures, and product labeling. This research aids in the identification of a potential predicate tool that shares your product's intended utility and technological characteristics.
Creating a comparative table becomes a foundational step, leading to a deeper dive into the Summaries of Safety and Effectiveness Data (SSEDs) on the FDA’s 510(k) database. This assessment distinguishes the similarities and differences that are crucial in the establishment of substantial equivalence to a predicate device.
It is also crucial to stay alert about the privacy of entries. Any electronic comments and attachments sent to the FDA are subject to public disclosure. Hence, applicants must ensure that no sensitive personal or proprietary business information is included unless intended for public view. For comments that include confidential data, a written or paper response is the appropriate channel, strictly adhering to the outlined 'Instructions for Written/Paper Submissions.'
Staying current with the FDA's role is imperative. The organization guarantees the safety, effectiveness, and security of medical equipment and is responsible for the welfare of public health. Understanding the agency's expectations and following their guidance documents is non-negotiable for a successful submission. The real challenge lies in compiling the necessary information within the set time frame, confident that it meets the FDA’s criteria for a determination of substantial equivalence.
In the rapidly evolving regulatory landscape, where there is a drive for accelerated pathways and enhanced collaboration, understanding the intricate dynamics of medical instrument categorization by the FDA is essential. The range of devices varies from low-risk class one to high-risk class three, where class one and two instruments undergo a simpler process like 510(k) clearance, while class three devices, which make up approximately 10% of FDA-regulated equipment, necessitate a more rigorous evaluation.
In summary, a strategic, well-informed, and meticulously planned approach to the 510(k) submission can greatly simplify the process, ensuring a successful regulatory pathway for medical equipment.
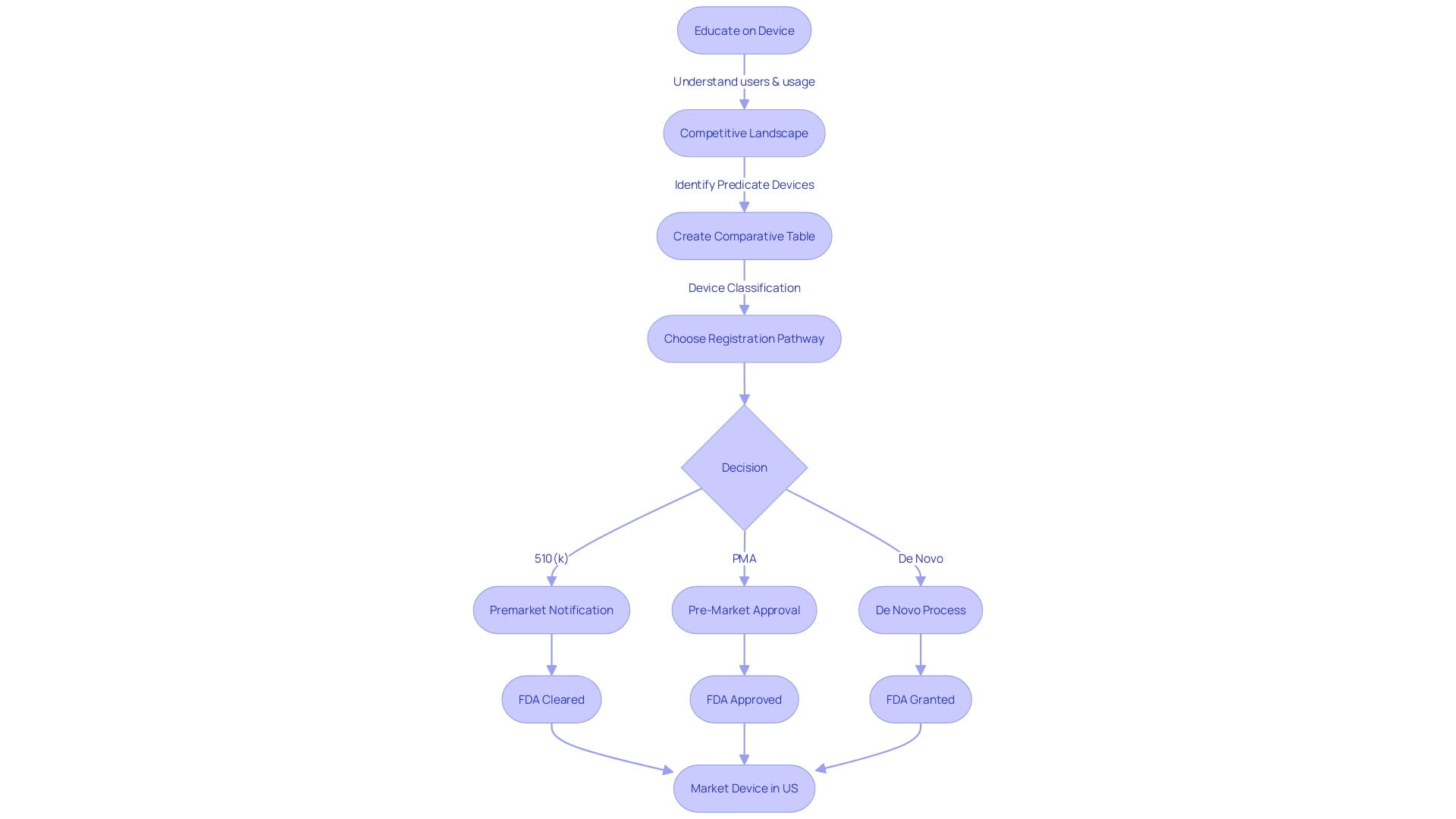
Timeline and Review Process for 510(k) Submissions
The FDA's 510(k) clearance process is a fundamental pathway for bringing medical instruments to market, with a structured timeline and review that developers must navigate adeptly. It starts with a thorough understanding of the apparatus in question, its intended use, and the users it serves, including clinicians, patients, and other healthcare professionals. Detailed instructions for use, warnings, and precautions are crucial elements of the document.
In order to prepare a robust 510(k) application, it is essential to identify predicate instruments that have already been cleared by the FDA, with similar intended use and technological characteristics. A comparative table showcasing the similarities and differences between your apparatus and the predicate is crucial. This involves reviewing resources such as research literature, clinical studies, and existing product information. The FDA's publicly accessible databases, including the Summaries of Safety and Effectiveness Data (SSEDs), are invaluable for this purpose.
The evaluation of your 510(k) application by the FDA involves several stages, where the agency ensures the safety, efficacy, and security of the medical device. As per recent updates from the FDA, the review process also includes guidelines to present information in a clear, conspicuous, and neutral manner, a practice exemplified by the new standards for direct-to-consumer prescription drug advertisements.
When providing comments or additional information to the FDA, it's crucial to adhere to their guidelines for submitting carefully. Publicly posted comments should exclude confidential information, such as personal medical details or sensitive business data. For comments containing confidential information, the FDA provides specific instructions for written or paper documents.
The entire review process is governed by the FDA's current thinking and guidelines, which are not legally enforceable but serve as recommendations unless tied to regulatory or statutory requirements. Comprehending and following these guidelines is crucial for a successful 510(k) process.
Since the FDA is accountable for not just medical instruments but also for the nation's food supply, cosmetics, and other consumer products, the agency's comprehensive approach guarantees the health and safety of the public. This overarching responsibility highlights the importance of a meticulous and informed 510(k) process.
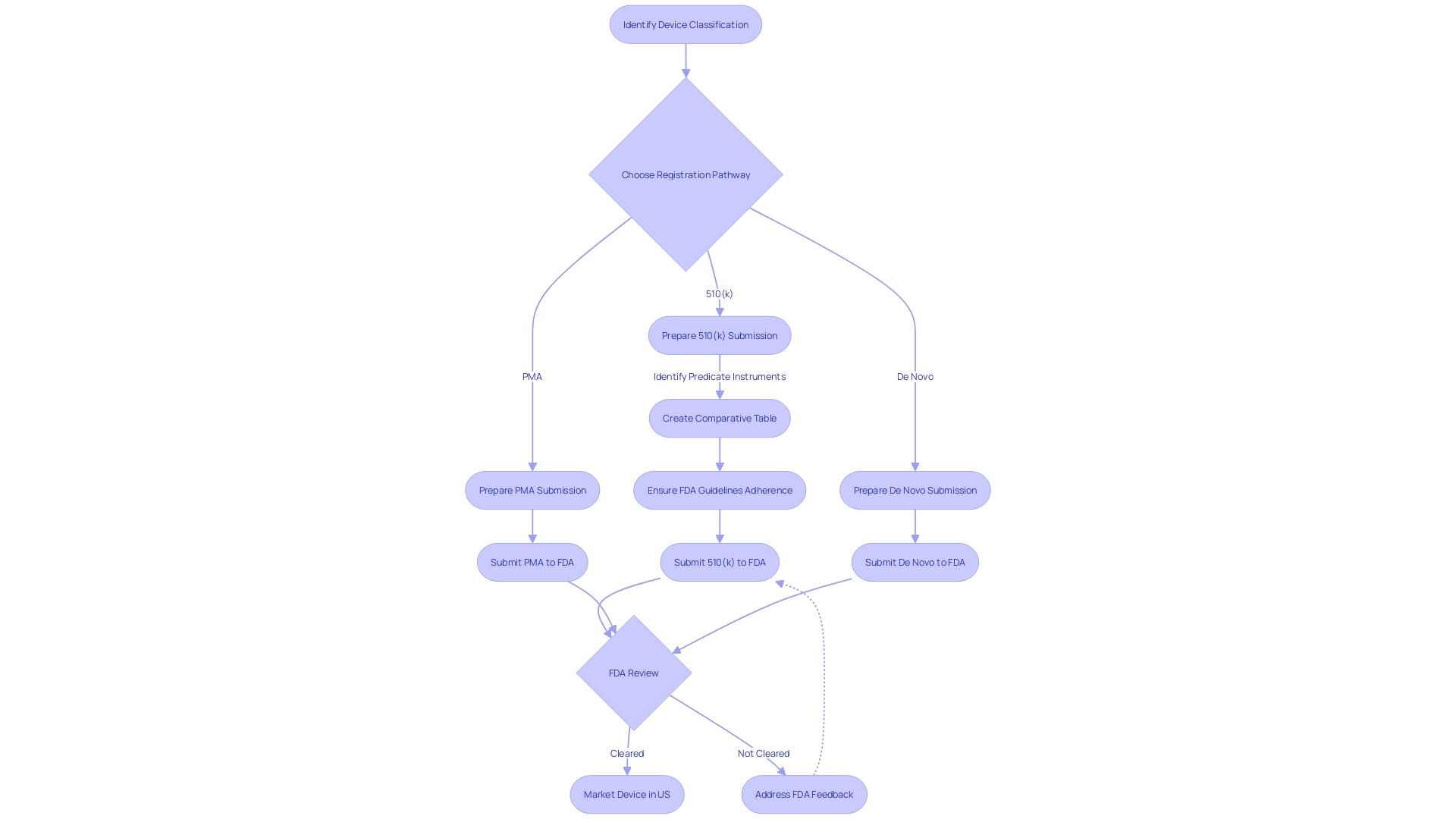
Common Pitfalls and Mistakes in 510(k) Submissions
To effectively navigate the 510(k) pathway, a thorough comprehension of the item at hand is crucial. This involves a thorough grasp of its users, ranging from clinicians to patients, and an in-depth review of its usage instructions, inclusive of warnings and precautions. Collaborating with the Marketing team is beneficial to gain insights into the competitive landscape. Examining materials like research literature, clinical studies, and competitor marketing assets will help in identifying a suitable predicate with similar intended use and technological features. Creating a comparative table is a strategic step in this process.
Moreover, the significance of familiarizing oneself with the Summaries of Safety and Effectiveness Data (SSEDs) available in the FDA's 510(k) database cannot be overstated. This enables a crucial assessment of the resemblances and differences between your apparatus and the identified precedent, which is a fundamental element of the procedure.
A clear, well-prepared 510(k) document is your conduit to contributing to the FDA's mission of ensuring the safety and efficacy of medical devices, and prioritizing public health. It is vital to pay attention to the FDA's guidelines, as outlined on their website, and comprehend the framework for presenting. A carefully organized document should include all necessary information, structured according to the FDA's expectations, to establish substantial equivalence.
The challenges frequently faced in the process of submitting, like insufficient descriptions of the apparatus or incomplete data from testing, can be overcome by following these strategies. The aim is to create a document that is as transparent and comprehensive as feasible, thereby preventing any delays or refusals. Keep in mind, the clarity of your submission directly affects the FDA's capability to evaluate your product effectively, which in turn influences the timeline of your product reaching the market and serving the needs of users.
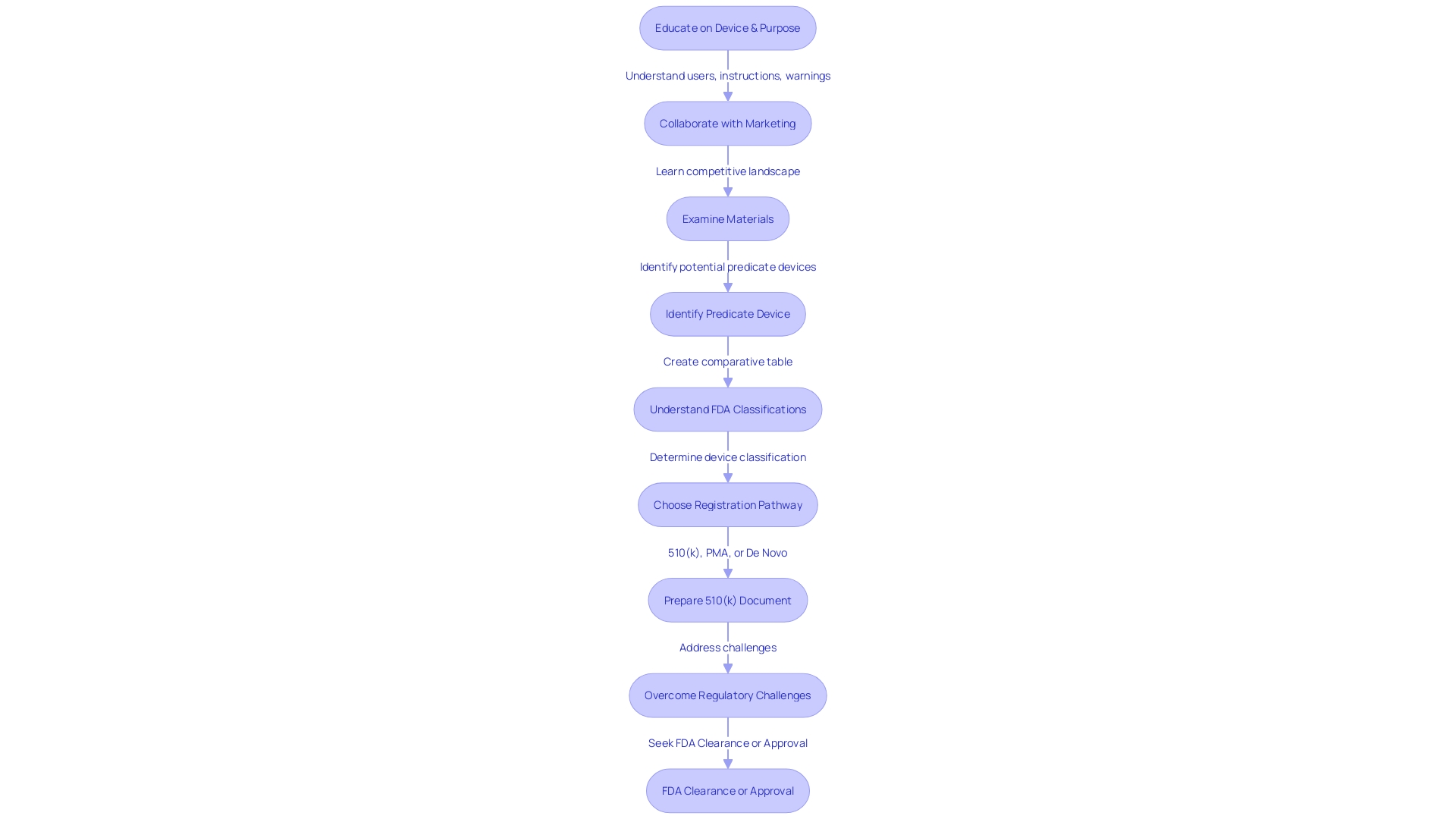
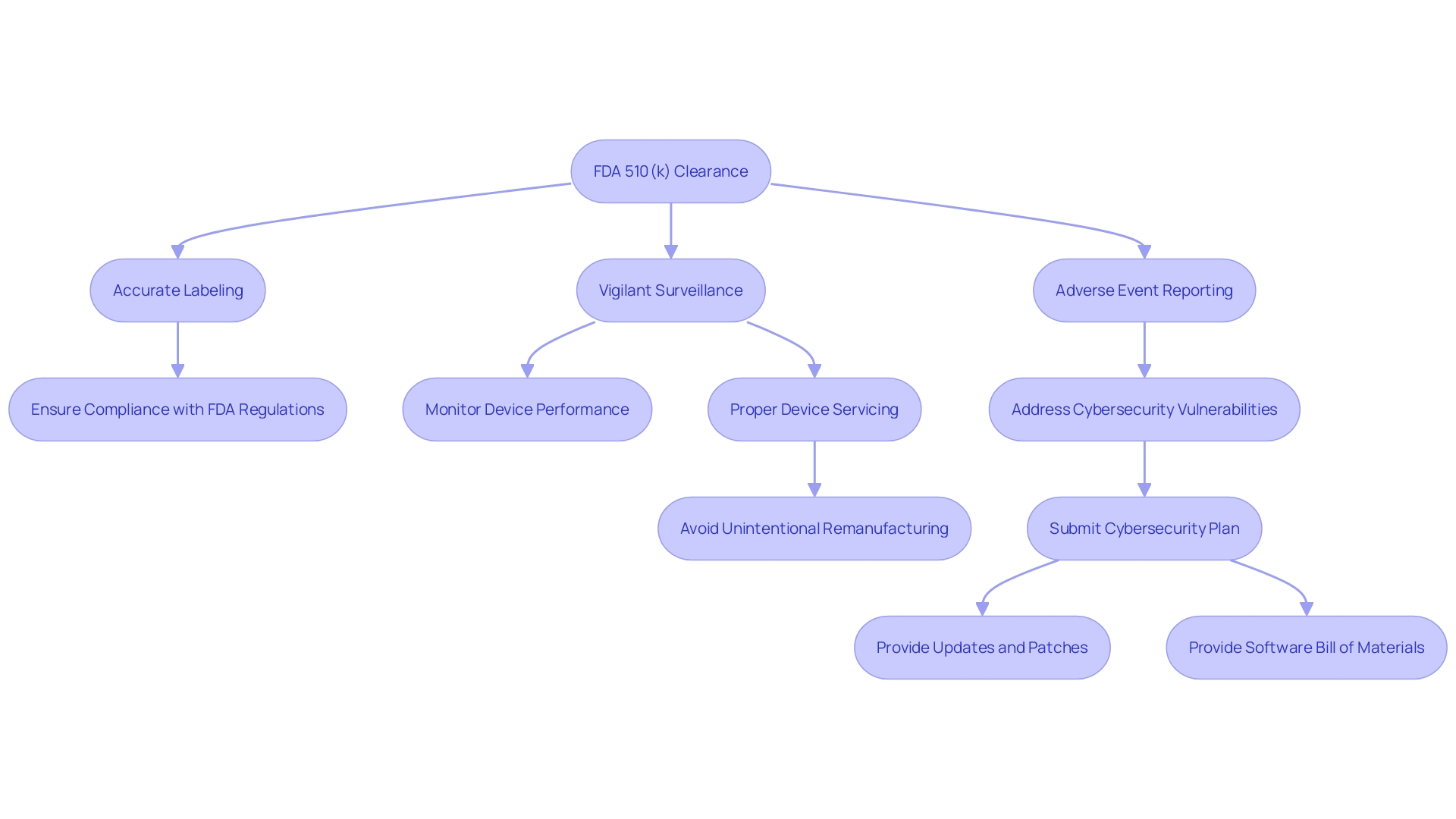
Additional Requirements for Marketing a 510(k) Cleared Device
Attaining FDA 510(k) clearance is a milestone for medical instrument manufacturers, but it's only the start of a meticulous post-clearance phase that requires careful attention to regulatory obligations. Manufacturers must navigate a complex landscape of post-market responsibilities, ensuring that their products are not only safe and effective but also meet the FDA's ongoing compliance requirements.
Labeling and advertising for medical instruments are subject to FDA scrutiny, where each apparatus must be accompanied by information that is truthful, non-misleading, and fully compliant with FDA regulations. This includes a clear classification name and product code, as well as a representative sampling of advertisements that accurately reflect the item's intended use without overstating its capabilities.
Post-market surveillance is crucial, as it involves the continuous monitoring of performance once it is in the hands of users. The FDA's active surveillance system aims to identify potential safety concerns that may arise from various products, including simple surgical masks and complex implantable pacemakers. This system is a vital part of the FDA's approach to safeguard public health, as demonstrated by the agency's initiatives to connect over 1.7 million injuries and 83,000 deaths in a 10-year span to medical equipment, emphasizing the crucial importance of vigilant post-market supervision.
Manufacturers are also obligated to report adverse events, a process that guarantees the FDA is promptly notified of any serious malfunctions or safety concerns associated with a medical product. With the FDA's High Risk List highlighting the oversight of medical equipment safety since 2009, the onus is on manufacturers to rigorously report any incidents that could impact the well-being of patients.
The recent FDA final rule on direct-to-consumer prescription drug advertisements underscores the agency's commitment to clear and neutral presentation of information, setting standards that extend to the medical equipment sector. These rules mandate that advertisements in television and radio formats present information in a consumer-friendly language, with sufficient duration and dual modality to ensure that the content is easily understandable and accessible.
In summary, after obtaining 510(k) clearance, manufacturers must adhere to a multifaceted post-market strategy that encompasses accurate labeling, vigilant surveillance, and thorough adverse event reporting, all within the framework of FDA's stringent regulatory landscape designed to safeguard public health.
Case Study: Successful 510(k) Submission Example
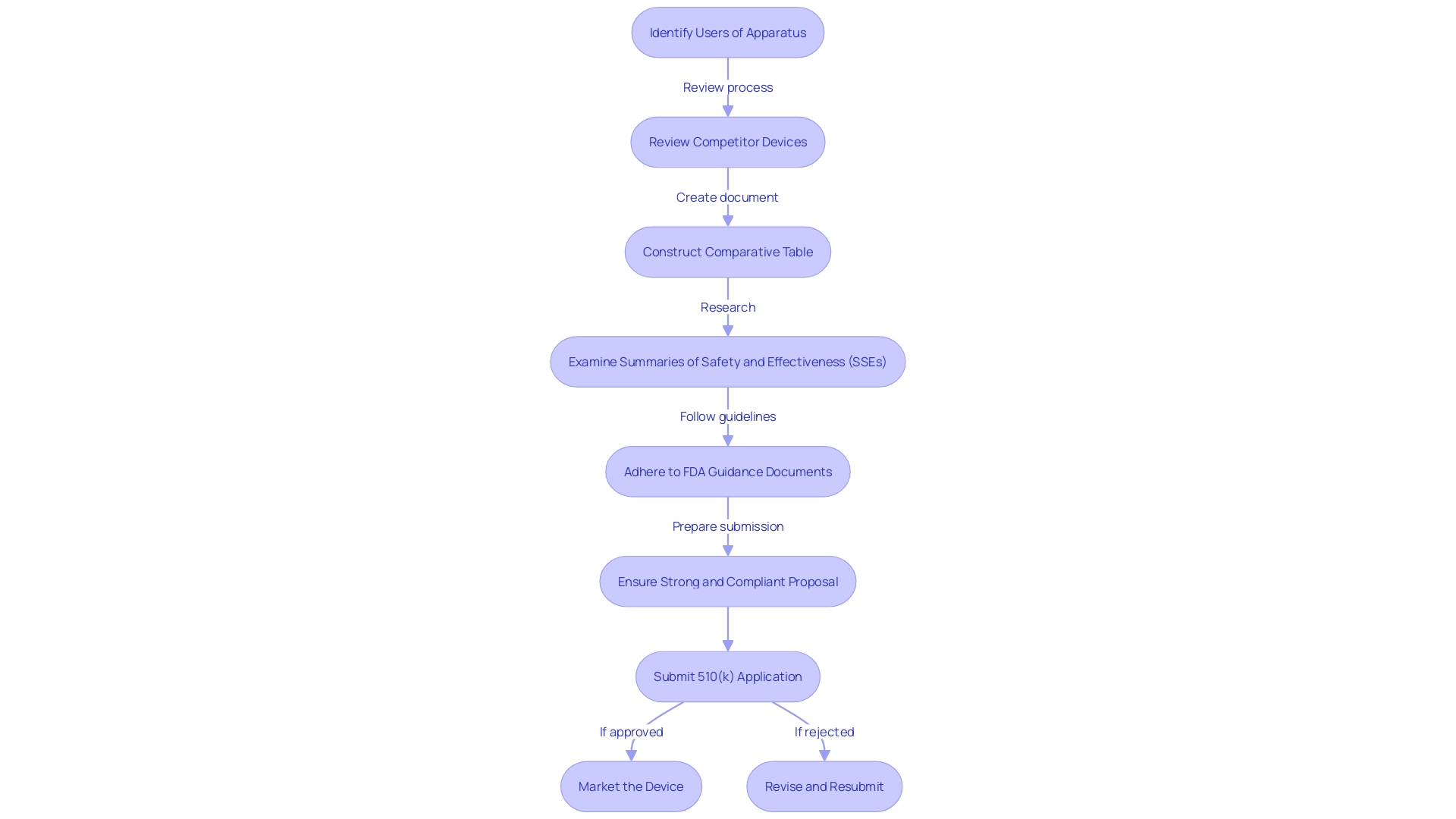
Exploring a real-world application, we delve into the journey of a medical equipment manufacturer who navigated the 510(k) submission pathway with exceptional strategy and careful planning. Their approach included a comprehensive understanding of the apparatus's users, ranging from clinicians to patients, and a thorough review of competitor devices. By constructing a comparative table that emphasized the resemblances and distinctions with potential predicate instruments, they were able to demonstrate substantial equivalence to the FDA. Key to their success was a thorough examination of Summaries of Safety and Effectiveness (SSEs) and adherence to FDA guidance documents, ensuring their proposal was strong and compliant. The case study of PharmaSens exemplifies this, marking a significant milestone in insulin pump technology with their patient-centric 'niia essential' system. Their achievement showcases the importance of a user-friendly design and the ability to meet unmet needs in diabetes management. The company's strategic planning and execution resulted in the successful clearance of their product, offering a model for others to emulate in the intricate 510(k) application procedure.
Best Practices for Navigating the 510(k) Process
Navigating the 510(k) submission and approval process for medical equipment requires a strategic approach, grounded in a thorough understanding of the apparatus, its users, and the regulatory landscape. A crucial first stage involves acquiring a comprehensive understanding of the purpose of the instrument, the healthcare professionals or individuals, such as doctors, dentists, or patients, who will utilize it, and the precise usage instructions, which encompass any advisories or cautions linked to the instrument. In addition, it is crucial to identify potential predicate instruments that have the same intended use and possess similar technological characteristics. This can be achieved through diligent research, including reviewing clinical studies, competitor websites and literature, and analyzing labeling and instructional materials. Developing a comparative table can aid in a clear visualization of similarities and differences between your product and existing predicates on the market.
Moreover, understanding the competitive landscape is vital. This involves examining rival products and promotional materials to determine the relative position of your product. Resources like the FDA's Summaries of Safety and Effectiveness Data (SSEs) available in the 510(k) database provide invaluable insights into predicate devices, allowing manufacturers to evaluate technological parity and address any potential safety and effectiveness concerns.
The FDA's guidance documents offer manufacturers a roadmap for what is expected in a 510(k) application. It is paramount for manufacturers to adhere to these recommendations, as they elucidate the necessary information and the process to follow for a submission to be accepted by the agency. The challenge lies in compiling the required information within the desired timeframe and ensuring the data aligns with the FDA's expectations to achieve a determination of substantial equivalence.
The medical industry plays a crucial role in healthcare by providing tools that assist in the diagnosis, treatment, and improvement of patient quality of life. Objects vary from basic tools like tongue depressors to intricate machinery used in medical testing and prosthetic equipment. The creation and advancement of these tools constitute a substantial portion of mechanical engineering, highlighting the convergence of technology and healthcare.
In light of the FDA's 510(k) modernization initiative, the agency has focused on enhancing the efficiency of the review process. While there have been discussions about the use of older predicates, the agency has recognized the benefits of long-term safety data associated with them. Public feedback has led to the development of best practices for selecting predicates, which the FDA has outlined in a draft guidance document released in September 2023. The initial stage in selecting a predicate involves confirming that the apparatus is lawfully marketed and presently registered with the FDA. The subsequent step requires an assessment to ensure the intended use aligns and that any differing technological characteristics do not raise new safety or effectiveness questions.
Given the complexities involved, device manufacturers must approach the 510(k) process with a well-informed and meticulous strategy to successfully navigate the regulatory pathway and ultimately bring their medical devices to market.
Conclusion
The FDA 510(k) submission process is a crucial gateway for medical device manufacturers to bring their products to market. It prioritizes patient safety by establishing substantial equivalence to an existing device already on the market, without the need for extensive clinical trials. To ensure a successful submission, a deep understanding of the device, its intended users, and the competitive landscape is vital.
The correct classification of medical devices is essential for determining the appropriate registration pathway. Thorough research and careful selection of predicate devices are necessary to demonstrate substantial equivalence. A comprehensive comparative analysis, including evaluating Summaries of Safety and Effectiveness Data (SSEs) on the FDA's 510(k) database, is crucial for understanding the similarities and distinctions between devices.
Confidentiality is paramount when submitting comments or documentation to the FDA. Following the detailed written/paper submission process is necessary to protect sensitive information. Adhering to FDA guidelines and understanding their expectations are non-negotiable for a successful submission.
The FDA's role in ensuring the safety and efficacy of medical devices underscores the need for rigorous compliance in submissions. Post-market responsibilities, such as accurate labeling, vigilant surveillance, and thorough adverse event reporting, are crucial to meet ongoing compliance requirements.
In conclusion, a well-informed and meticulously planned approach to the FDA 510(k) submission process ensures safety and efficacy while bringing medical devices to market. By prioritizing patient safety and following FDA guidelines, manufacturers can contribute to the overall goal of protecting public health.
Start your FDA 510(k) submission process today and ensure the success of your medical device.




