Introduction
In the realm of medical technology, predicate devices serve as the benchmark for assessing the safety and effectiveness of new medical devices. These devices, previously cleared by the FDA, act as a comparative standard for new devices seeking approval through the 510(k) process. The FDA's 510(k) modernization efforts have led to draft guidance outlining best practices for selecting appropriate predicate devices.
This guidance emphasizes the importance of choosing predicates that are legally marketed and share the same intended use as the new device, without introducing new safety and effectiveness concerns. By following these guidelines, medical device manufacturers can ensure technological relevance, regulatory compliance, and harness the benefits of older predicates, such as the accumulation of long-term safety data. Transparent communication about a device's intended use, development, performance, and logic is also crucial for enhancing its explainability to healthcare professionals and patients.
In this article, we will explore the types of predicate devices, criteria for selecting a predicate device, and the importance of safety, effectiveness, and well-established methods in the medical device industry.
What are Predicate Devices?
In the field of medical technology, reference products serve as the standard for evaluating the safety and efficacy of novel medical devices. A previous apparatus is usually one that has been previously cleared by the FDA, acting as a comparative standard for a new apparatus seeking approval through the 510(k) process. The efforts made by the FDA to modernize the 510(k) process have resulted in the creation of draft guidance that highlights the most effective methods for choosing appropriate reference instruments. This guidance highlights the significance of selecting products that are lawfully marketed and have the same intended purpose as the new equipment, without introducing additional safety and effectiveness considerations. The FDA supports the selection of examples cleared through established methods, like FDA-recognized consensus standards, guidance documents, or qualified tools for developing healthcare devices. This organized method aims to utilize the advantages of previous conditions, like the valuable gathering of long-term safety data, while guaranteeing technological relevance and regulatory compliance. Furthermore, for a thorough comprehension of a healthcare instrument, it is crucial to openly communicate about its intended purpose, advancement, effectiveness, and reasoning, improving its clarity for healthcare practitioners and patients alike.
Types of Predicate Devices
In the realm of medical equipment regulation, the distinction between prototype tools is a fundamental concept with two primary categories: preamendments prototypes and postamendments prototypes. Preamendments products refer to those that were available before the Medical Device Amendments of 1976, and they are generally subject to performance standards issued by the FDA. Postamendments instruments, on the other hand, entered the market after these amendments and must undergo a more rigorous premarket approval process or adhere to specific classifications and controls.
The FDA uses classification names to categorize items based on their intended use and risk level, guiding manufacturers through the regulatory landscape. Each piece of equipment receives a product code that corresponds to its generic category, simplifying the identification process for regulatory purposes.
When it comes to advertising and other promotional materials, the FDA mandates that a representative sampling of these materials accurately reflects the promotional claims made for it. Any significant alteration in labeling or advertising that may affect the identity or the safety and effectiveness of the product initiates regulatory examination. This includes changes to the name, ingredients, intended use, contraindications, warnings, or instructions of the product.
It is also crucial for initial importers, who advance the marketing of products from foreign manufacturers to the end consumer, to comprehend that repackaging or relabeling can have regulatory implications and must be carried out in compliance with FDA standards.
The FDA's focus on ensuring that healthcare instruments meet strict safety and effectiveness criteria reflects its broader mission to protect public health. This dedication extends to guaranteeing appropriate gadget servicing and remanufacturing practices, which are crucial for the longevity and efficacy of gadgets, particularly those that are reused, such as infant warmers and ventilators.
The recent guidance from the agency regarding remanufacturing versus servicing of healthcare equipment highlights the significance of following the safety and performance specifications set by the original equipment manufacturer. This advice is especially relevant considering the intricate and diverse technologies used in today's healthcare equipment.
Additionally, the role played by the FDA in promoting innovation and establishing criteria for healthcare equipment is vital. The creation of the Digital Health Advisory Committee demonstrates the FDA's proactive approach to regulating emerging technologies and ensuring that digital health tools are safe and effective for users.
As the medical equipment market continues to expand and innovate, regulations evolve to match the pace of technological advancements. Manufacturers and stakeholders must remain vigilant and informed about these regulations to maintain compliance and contribute to the delivery of quality patient care.
Preamendments Devices
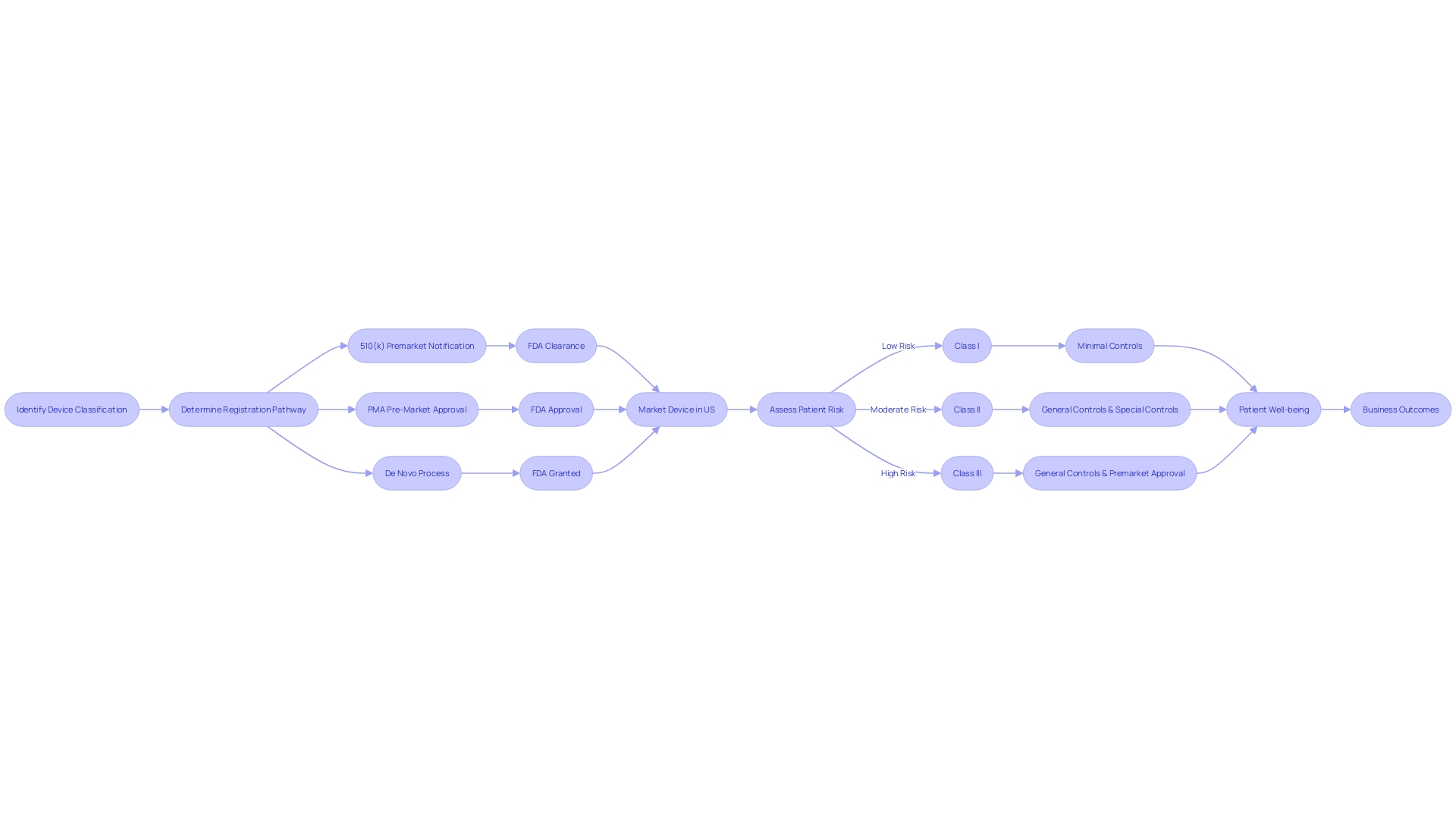
Medical instruments introduced prior to the implementation of the Medical Device Amendments in 1976 are referred to as preamendments instruments. These legacy products are not bound by the contemporary obligation of submitting a premarket notification, also referred to as a 510(k), to the FDA for clearance. To navigate the regulatory framework effectively, it is crucial to comprehend the FDA's classification system, which categorizes medical instruments into three classes based on the risk they pose to patients. Class I products carry the lowest risk, whereas Class III products, such as life-supporting implantables, have the highest risk and necessitate more comprehensive regulatory review. Preamendments products belong to a unique category where their historical utilization in healthcare enables them to be marketed without the 510(k) procedure, as long as there are no substantial modifications to their design, intended use, or safety profile that would require a review.
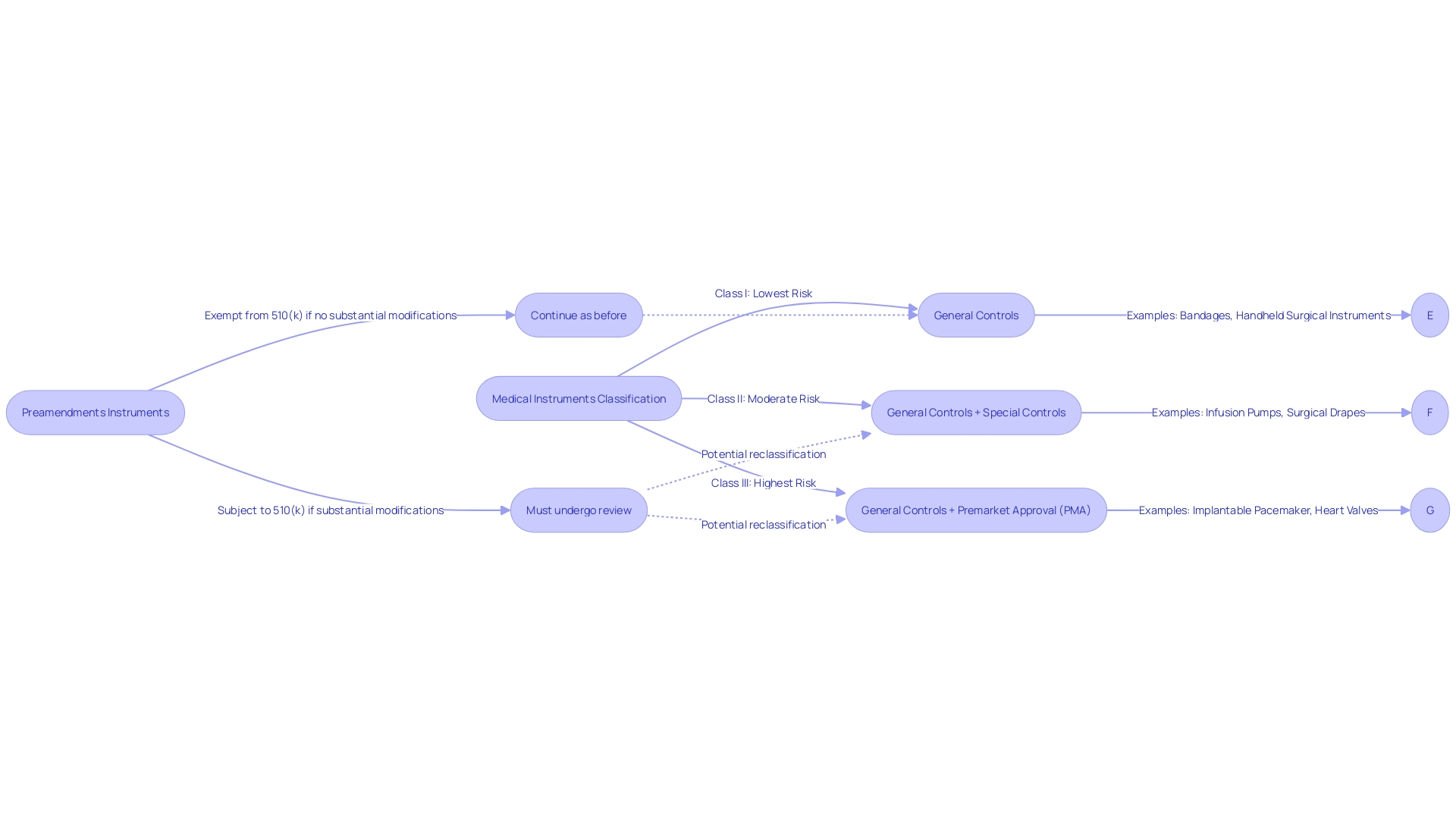
Postamendments Devices
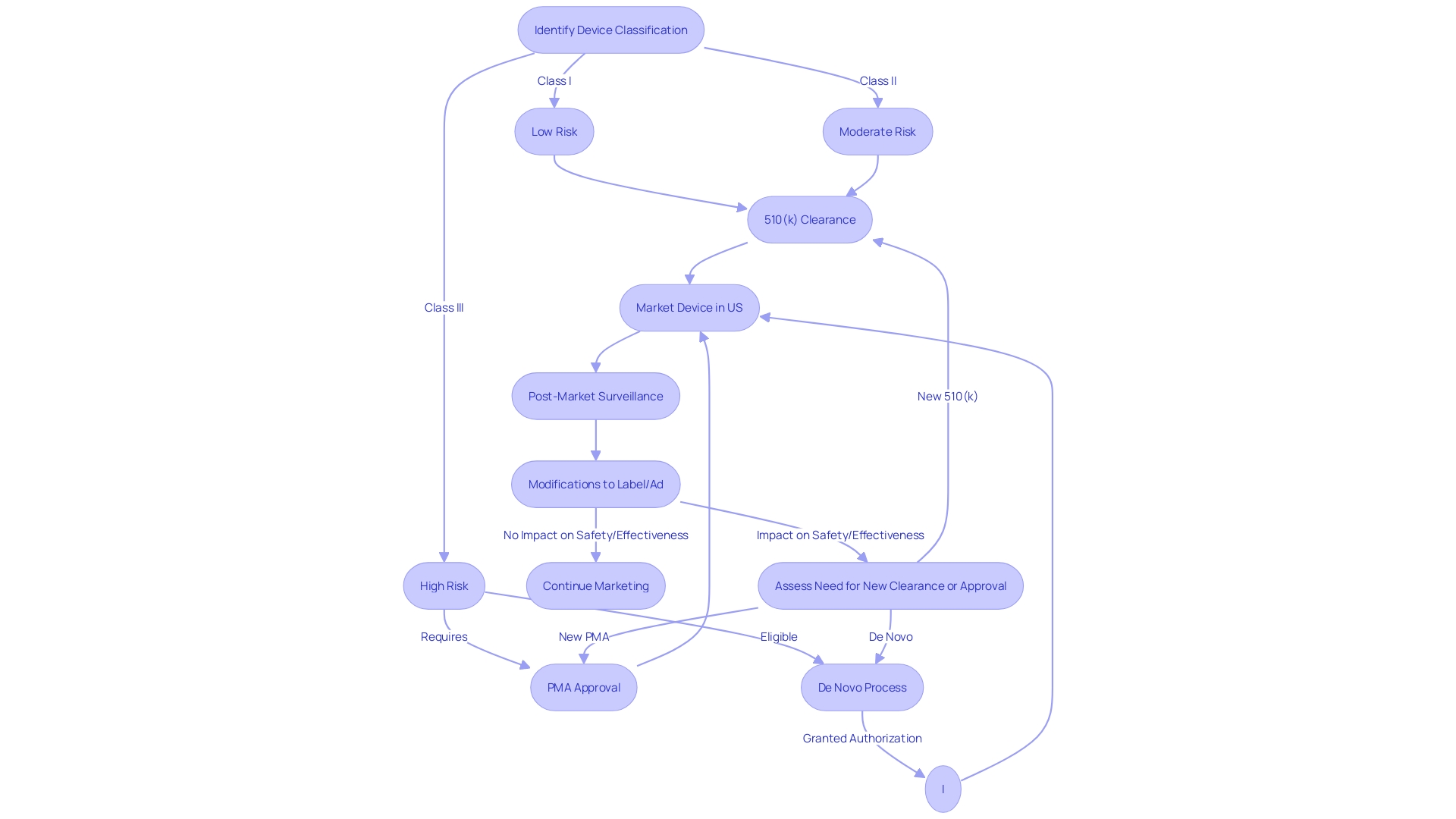
Devices that emerged post the 1976 Medical Device Amendments are subject to stringent FDA oversight. They require a premarket notification, commonly known as a 510(k) clearance, establishing their substantial equivalence to an existing predicate product. This procedure is crucial in guaranteeing that new medical equipment is as secure and efficient as their equivalents already accessible to patients.
As an example, the FDA's reclassification of ultrasound cyclodestructive instruments from class III to class II was met with broad support, highlighting the balance between strict initial regulation and subsequent easing of controls. This change aims to strengthen patient access to these tools without jeopardizing safety.
Moreover, the FDA's role extends beyond initial clearance, influencing coverage and reimbursement decisions by various payors. Despite FDA clearance, there may be delays in coverage which can affect patient access to the latest medical equipment. The FDA categorizes equipment into three classifications, with class III equipment, such as life-supporting implantables, undergoing the most rigorous approval process and comprising roughly 10% of FDA-regulated equipment.
It's important to note that modifications to the labeling or advertisements that impact a product's identity or safety necessitate careful consideration, as these can have substantial implications for the classification and regulatory status of the product.
Recent efforts to streamline regulatory pathways, especially in the wake of the COVID-19 pandemic, underscore the importance of collaboration between agencies and industry to facilitate quicker access to healthcare technology that caters to pressing medical needs, particularly in innovative fields like digital health and personalized medicine.
Criteria for Selecting a Predicate Device
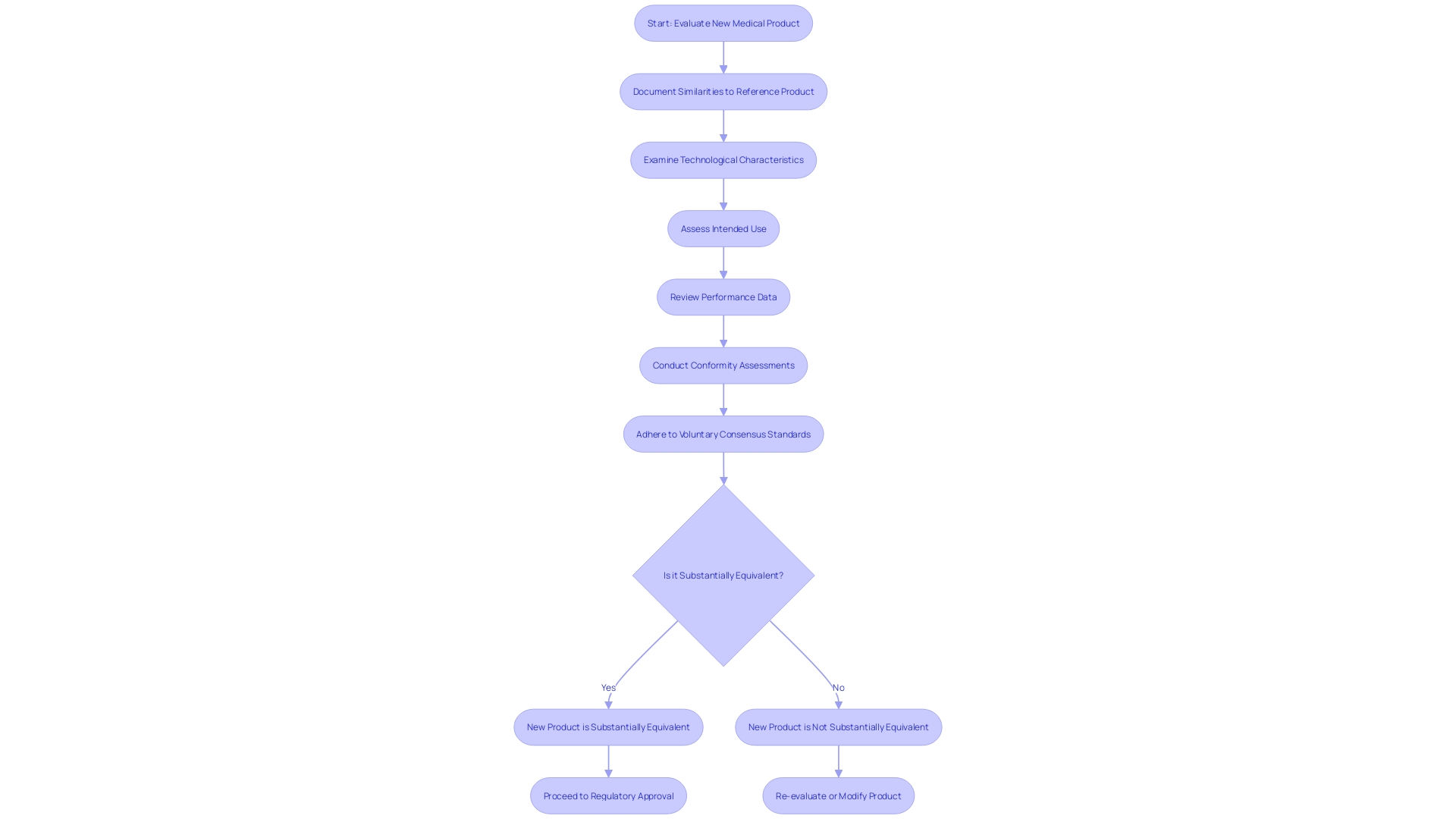
Choosing a suitable alternative component is an essential aspect of the 510(k) submission procedure for manufacturers of medical devices. It requires a thorough evaluation of potential products that have already been approved and registered by the FDA. The selected term must have the same intended purpose as the new apparatus, and any technological disparities should not raise new concerns about safety and efficacy. Moreover, the predicate should ideally exhibit sensory design elements, such as affordances and semantics, which can be perceived through visual, tactile, and auditory cues. This is crucial for healthcare equipment because they must instinctively convey their utilization to the operator, thereby lessening the probability of user mistake, which could have severe or even life-threatening outcomes.
In the context of medical equipment development, integrating sensory design not only contributes to user-friendliness but also ensures the product conveys cleanliness, reliability, and functionality over time. For example, the design of a speculum, a device used during pelvic exams, has evolved to be less intimidating and more comfortable for the patient, reflecting the importance of user experience in device design.
Taking into account these factors, the FDA has started the 510(k) modernization initiative, which involves creating guidelines for choosing comparable products. This initiative was propelled by public feedback and the release of a draft guidance document on September 7, 2023. The document emphasizes the advantages of using older predicates, such as the accumulation of extensive safety data, which can be an asset to the 510(k) review process.
Furthermore, the digitization of healthcare equipment is a swiftly expanding area, where a profound comprehension of information and its significance in the development and adherence to regulations is crucial. This transformation is not only about converting documents into digital formats but also about leveraging digitalization to optimize the route to market and compliance processes.
The healthcare equipment market is extremely competitive, and a successful new product launch is indicative of a company's capability to navigate from concept to market entry. Therefore, it is imperative for stakeholders to choose a development partner with a full spectrum of services, from concept generation to market launch, who also possesses technical expertise, regulatory knowledge, and an understanding of market dynamics. The merging of software and hardware components in healthcare technology is becoming increasingly crucial, providing companies skilled in this field a unique competitive edge.
Indications for Use and Technological Characteristics
In order to create a significant and reliable comparison between a novel healthcare instrument and its predecessor, it is crucial that they have similar indications for usage and technological features. This alignment is crucial as it informs healthcare professionals and regulatory bodies about the equipment's intended purpose and functionality within the healthcare workflow. It also plays a major part in the regulatory process, where the Food and Drug Administration (FDA) evaluates the safety and effectiveness of healthcare equipment. After FDA approval, the decision to cover and reimburse for the equipment lies with payors, who rely on this information to assess the equipment's value and impact on patient care. As stated by the FDA, the data submitted for safety and effectiveness may not always align with the data payors need, potentially leading to delayed patient access. As a result, clear communication of a product's intended purpose and its connection to current technologies is not just a regulatory necessity but also a promoter of seamless integration into the market and healthcare procedures, guaranteeing that patients can take advantage of the most recent healthcare advancements without unwarranted delays.
Design, Materials, and Performance Characteristics
Ensuring the safety and effectiveness of healthcare equipment is of utmost importance, considering the potential risks they present. When creating new medical products, it is crucial to establish a strong connection with an existing reference item. The predicate instrument serves as a benchmark, providing a basis for comparison in terms of design, materials, and performance characteristics. An efficient assessment of the safety and efficacy of the new apparatus depends on these similarities. This process is not only a mere formality but a rigorous assessment rooted in the reality of the impact healthcare instruments have on patient outcomes.
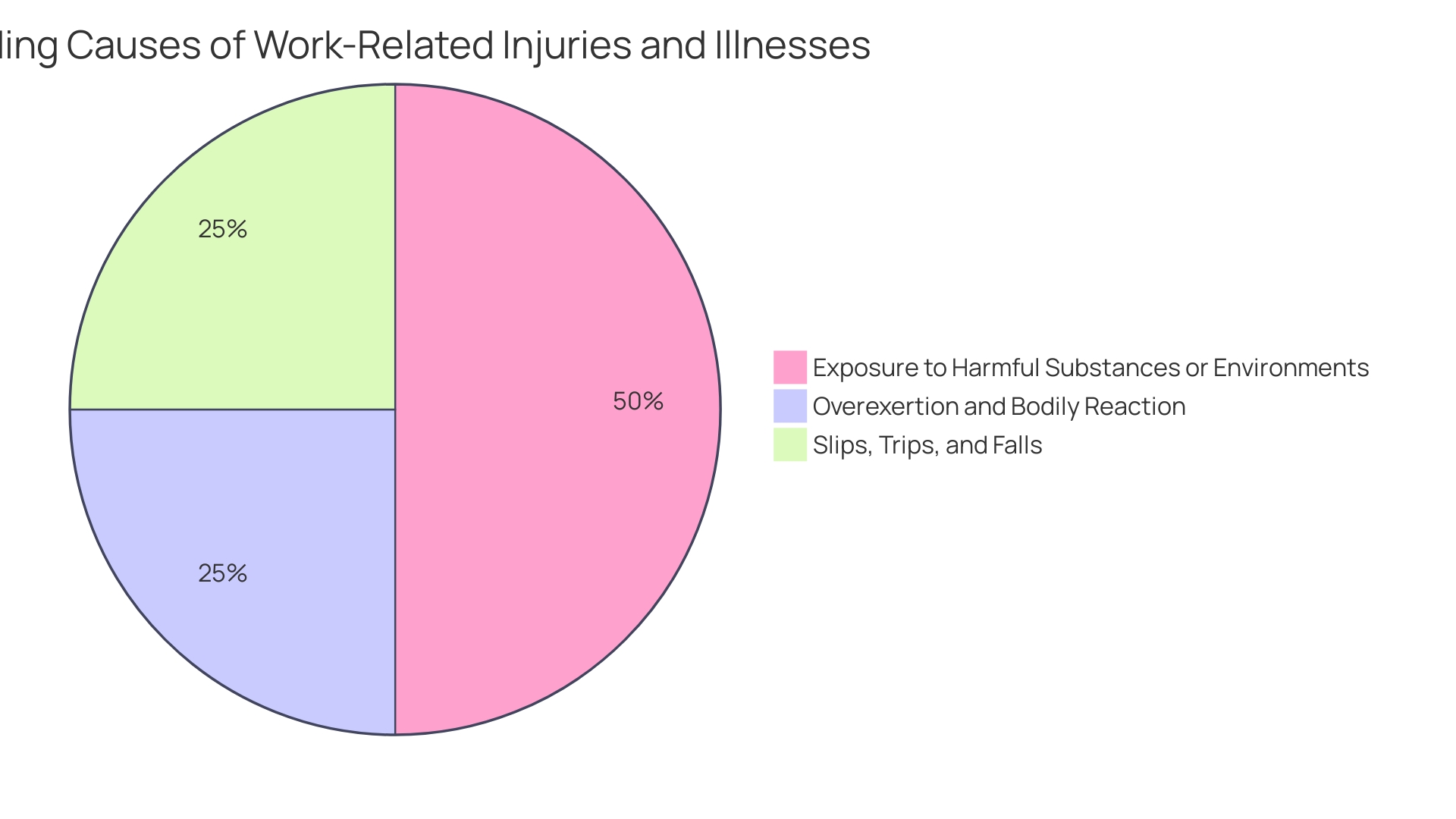
Based on an analysis of FDA information from 2018, within a decade, there were more than 1.7 million injuries and 83,000 fatalities in the United States that could be linked to healthcare equipment, which ranged from common items like surgical masks to intricate technologies such as implantable pacemakers. Such statistics underscore the importance of stringent regulatory oversight and the necessity for a robust postmarket surveillance system. The FDA has acknowledged the challenges in this area and is working on establishing an active surveillance system to monitor the safety of healthcare equipment. This project is in its early phases, concentrating on a chosen few gadgets, with intentions for growth, dependent upon overcoming barriers like financing and identifying gadget users.
Openness in information about healthcare equipment, including purpose, advancement, and efficiency, is crucial for all parties involved, such as doctors and healthcare personnel who have a significant impact on patient treatment. As emphasized in industry discussion, the 'logic' and 'explainability' of a mechanism's output or action are essential components of transparency, ensuring that risks and patient outcomes are clearly communicated and understood.
The development procedure of healthcare equipment must take into account not just the patient but also the wider network of users such as nurses, doctors, and maintenance staff. Service design, which is about designing for these varied stakeholders, is as critical as patient-centered design. The collaborative effort in patient care significantly influences patient conditions, highlighting the interconnectedness of equipment design and healthcare service delivery.
In the industry of healthcare equipment, where regulations are strict, the choice of a design collaborator is vital. The extent of expertise a company specializing in the design of healthcare equipment can offer is a determining factor for a successful partnership and outcome. Furthermore, an assessment of the biological aspects is a necessary stage prior to conducting clinical trials, underscoring the importance of a comprehensive evaluation of a healthcare product's biological safety for particular markets and populations.
In the end, the objective is to guarantee that healthcare instruments are not only groundbreaking but are also secure, efficient, and created with openness and customer focus as their foundation.
Safety and Effectiveness
Ensuring that a predicate instrument aligns with regulatory standards is crucial for establishing substantial equivalence with new medical devices. This not only relates to the safety and effectiveness of the product but also involves a comprehensive review of manufacturing practices and labeling. As per the Federal Food, Drug, and Cosmetic Act, a product must meet specific criteria regarding product code, classification name, and other factors, which are essential for FDA's classification process.
The importance of this process is underscored by the FDA's recent updates to the presentation of major statements in direct-to-consumer prescription drug advertisements, emphasizing the use of clear, conspicuous, and neutral language. This rule is a component of the FDA's continuous endeavor to guarantee the well-being of the public by protecting the safety and effectiveness of healthcare instruments.
In the past, the 510(k) clearance process has been a pathway for fast-tracked approval of products considered comparable to previously approved items already available in the market. However, it has faced scrutiny, as highlighted in the 2018 documentary 'The Bleeding Edge', which revealed instances where lack of clinical trials for approved equipment led to patient injuries. Lessons from such cases have prompted the FDA to reinforce the rigor of the clearance process.
In light of these events, the FDA continues to foster innovation while ensuring safety through the use of voluntary consensus standards. These standards are developed according to principles of transparency, openness, and due process, as defined by the ANSI Essential Requirements and OMB Circular A-119. The commitment to such standards supports the alignment of technological progress and guarantees the excellence and security of novel healthcare instruments.
Moreover, the Office of Readiness and Response's collaborative approach to standards development signifies the FDA's commitment to incorporating voluntary consensus standards into regulatory frameworks. This approach enables the FDA to drive the development and appropriate use of consensus standards, thereby promoting efficiency and quality in regulatory reviews.
Use of Well-Established Methods
Integrating proven approaches from the advancement of predicate tools into the formation of fresh healthcare apparatus is crucial for guaranteeing both dependability and uniformity throughout the assessment procedure. This approach aligns with user-centered design principles, which prioritize the needs, behaviors, and motivations of all potential users, including clinicians, patients, and hospital staff, to foster impactful and relevant experiences. For instance, trials involving the use of AI to enhance MRI image quality underscore the importance of integrating advanced technology with user-centric design to improve clinical outcomes.
Selecting a development partner with a comprehensive understanding of the lifecycle of healthcare devices—from concept generation to market launch—is crucial. This partner should demonstrate a proven track record of successful product launches and the ability to integrate software and hardware components, as these capabilities provide a competitive advantage in the digital health arena. Moreover, considering the FDA classifying equipment into three categories according to risk, it is crucial that developers possess a thorough understanding of regulatory intricacies to enable a seamless progression from idea to market, particularly for high-risk class three apparatus which constitute a substantial portion of FDA-regulated machinery and require comprehensive regulatory examination.
Best Practices for Choosing a Predicate Device
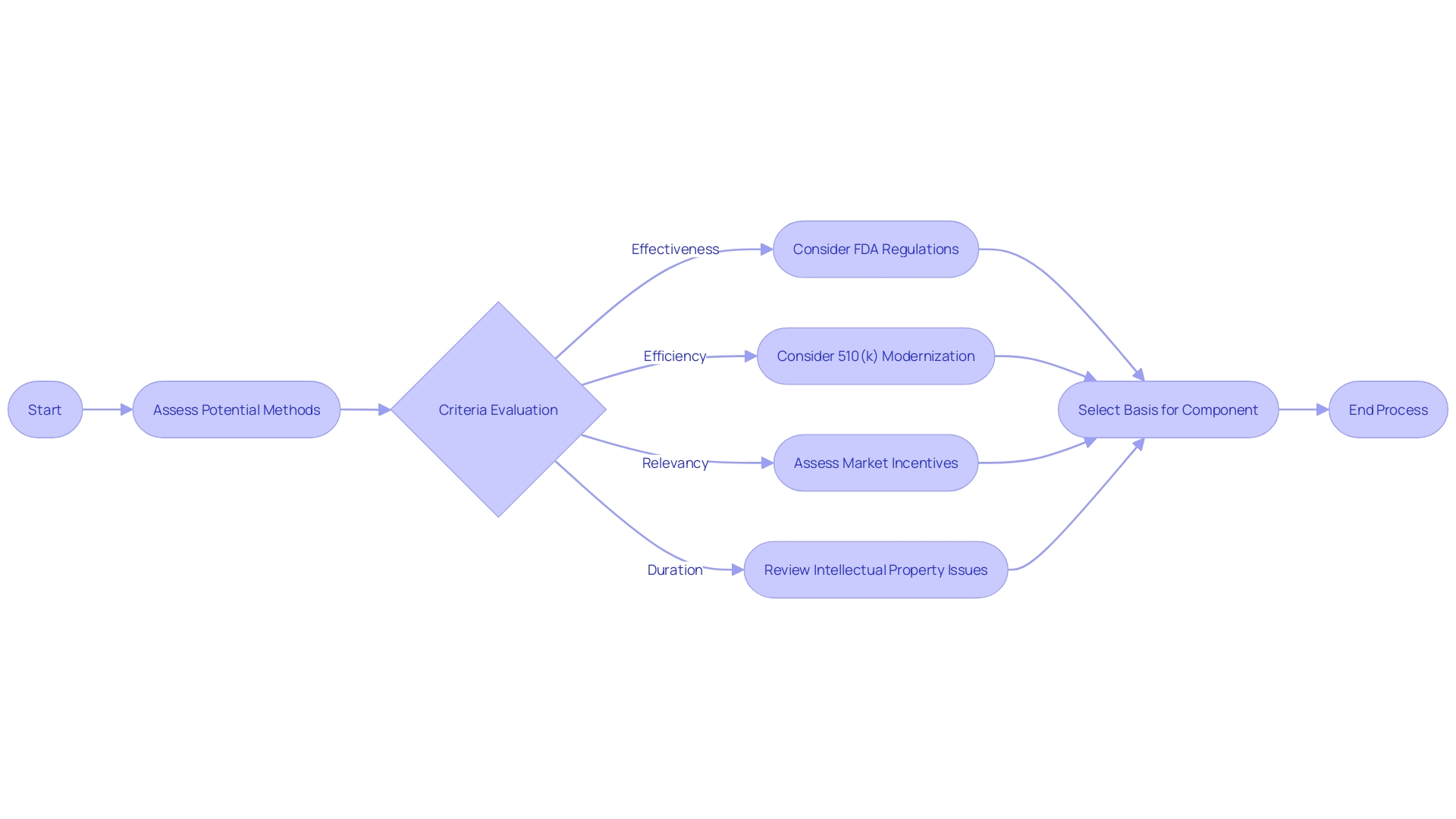
Choosing an essential component is a crucial procedure in the advancement and approval by regulators of innovative medical technologies. To ensure that the comparison of a new item to an existing one is both valid and reliable, it is essential to apply a rigorous set of criteria. Sugai and Horner (2006) propose four criteria for assessing potential methods: effectiveness, efficiency, relevancy, and duration. Effectiveness requires that the subject has proven utility, as ideally demonstrated in peer-reviewed research. However, many new technologies may not have extensive research available, and therefore, alternative methods such as examining available product information might be necessary.
Efficiency relates to the cost-benefit ratio of a particular apparatus. It is worthwhile to consider if the older device provides a solid track record of performance without excessive costs. The relevancy criterion assesses the applicability of the statement to the current technological and clinical landscape, ensuring that it meets contemporary standards and can still address current medical needs. Lastly, duration considers the sustainability of the subject's benefits over time.
Within the scope of FDA regulations, the agency's 510(k) modernization initiative has highlighted the significance of choosing legally marketed instruments as references and assessing their intended applications and technological features. Devices that raise new questions of safety and effectiveness are scrutinized carefully. The FDA's initial guidance document, published on September 7, 2023, outlines recommended approaches for selecting a basis, which involve utilizing FDA-approved agreement standards, FDA guidance documents, and qualified technological tools.
The FDA also advises on the use of widely accepted scientific methods and suggests that having more information about the previous reference can improve the selection process. Since the development of medical equipment covers a wide range of tasks from generating ideas to launching in the market, choosing a reference product is only a small aspect of a complicated journey to meet regulatory requirements. It is one that requires technical expertise, a deep understanding of regulatory landscapes, and a comprehensive approach to development that can save time, money, and enhance the project's success.
Devices Cleared Using Well-Established Methods
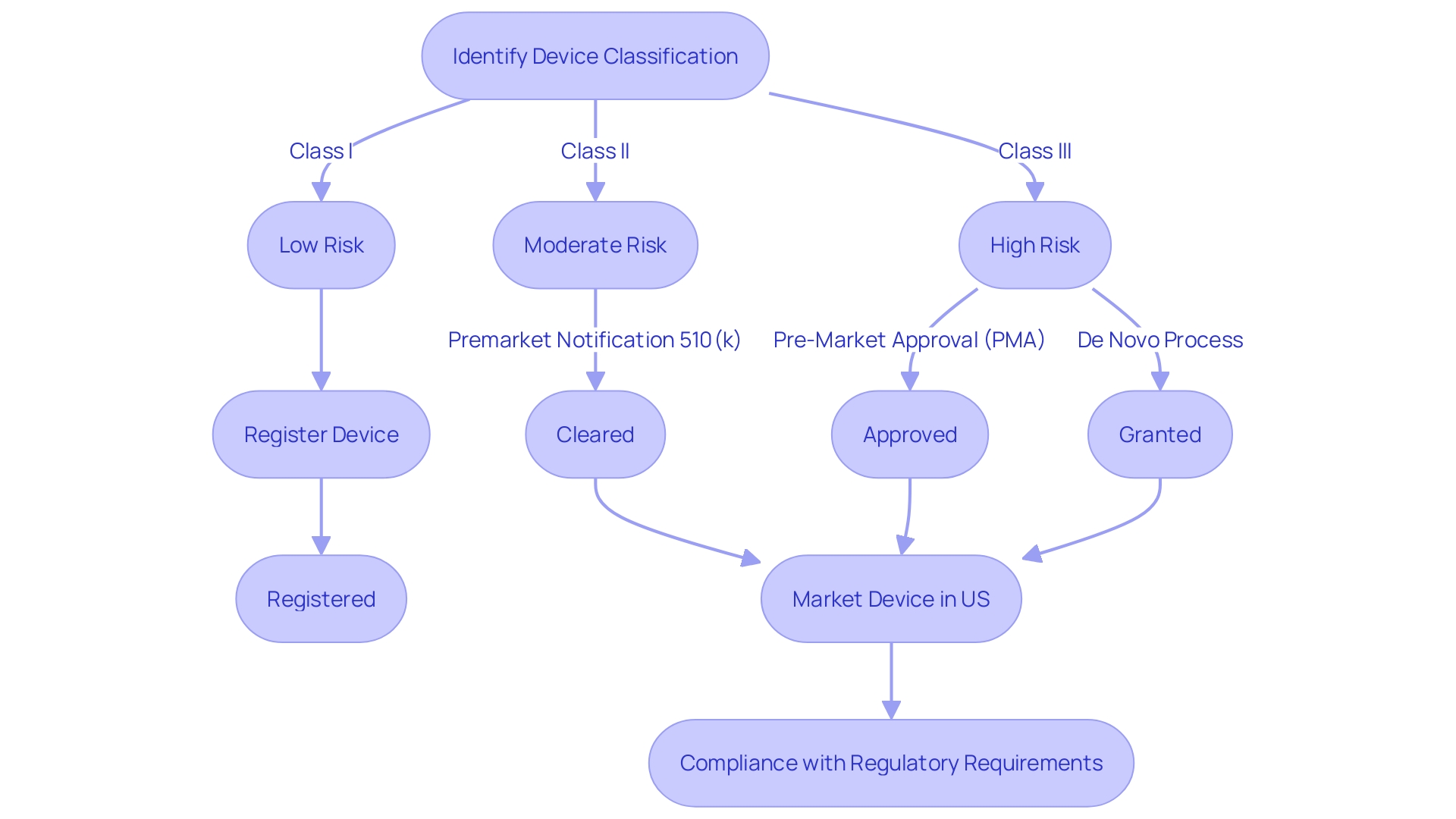
When choosing a predicate instrument for FDA clearance, it is crucial to navigate the intricate regulatory terrain of equipment classification and approval pathways. The FDA classifies medical equipment into three categories based on the level of risk they pose to patients. Each class has a corresponding registration pathway: Class I generally require Premarket Notification (510(k)), Class II may necessitate Pre-Market Approval (PMA), and Class III may be eligible for the De Novo process. These terms—Registered, Cleared, Approved, and Granted—carry distinct meanings and implications for regulatory professionals who must ensure their product meets the stringent criteria for market entry. Utilizing a predicate mechanism that has undergone thorough evaluation and adherence to consensus standards ensures not only compliance with regulatory requirements but also the mechanism's reliability. The FDA's dedication to public health is evident in its rigorous approval processes, which are intended to ensure the safety and efficacy of healthcare equipment. Comprehending these subtleties is essential for effectively launching a healthcare apparatus to the market while ensuring patient safety.
Devices That Meet or Exceed Expected Safety and Performance
When choosing a reference instrument for comparison, it is crucial to select one that meets or exceeds the expected safety and performance criteria of the new instrument. This strategic alignment is exemplified by the Impella Connect System, a combination of software and hardware designed to work with the Automated Impella Controller (AIC) in critical care settings for temporary ventricular support. The system notably includes functionalities like remote monitoring and color-coded alarm notifications, which are essential for detecting life-threatening conditions and providing timely alerts to healthcare providers. Such features, deeply rooted in software logic and transparency, are subject to premarket authorization to ensure they comply with regulatory standards.
Furthermore, the integration of voluntary consensus standards, as emphasized by UL Solutions, plays a pivotal role in maintaining regulatory quality and fostering innovation. For example, the deployment of the Compass™ project by the URMC involved a careful needs assessment and phased implementation across various clinical sites, integrating existing ultrasound systems and enriching education with Point-of-Care Ultrasound (POCUS).
This careful selection and implementation process, backed by transparency and conformity to consensus standards, is instrumental in addressing potential risks, including cybersecurity issues, while maintaining the pace of innovation. As Bijan Elahi, an experienced specialist in safety risk management for healthcare equipment, has instructed numerous individuals on optimal approaches, it is clear that the utilization of reference tools that fulfill strict criteria is essential to guaranteeing the safety and effectiveness of tools in clinical environments.
Devices Without Unmitigated Use-Related or Design-Related Safety Issues
When choosing a predicate instrument for your clinical research, it's important to consider its track record in post-market surveillance (PMS). Post-market surveillance (PMS) plays a crucial role in monitoring the safety and effectiveness of healthcare instruments after they've been approved and are in use in real-world environments. The process involves a variety of data collection methods, including passive surveillance like reports from healthcare professionals and patients, as well as active surveillance through registries and studies.
Recent statistics underscore the importance of this consideration. Over a period of ten years, a shocking 1.7 million injuries and 83,000 deaths in the United States were potentially associated with medical equipment, as indicated by a 2018 examination of FDA data. These figures highlight the critical need for continuous evaluation and reporting of instrument performance and the potential consequences of disregarding safety issues.
Furthermore, the design of an apparatus should accommodate the end-user, particularly for those intended for home use. The executive brief for 2024 emphasizes the importance of creating products with the lay user in mind to prevent misuse and harm. Regrettably, numerous home-use appliances lack this consideration, which can result in unfavorable outcomes.
According to Bijan Elahi, a well-known specialist in safety risk management for medical machinery with more than 29 years of expertise, 'Safety Risk Management for Medical Devices demystifies risk management, providing clarity of thought and confidence to the practitioners of risk management as they do their work.' He underscores the necessity of defining clear risk acceptance criteria and knowing when to halt risk reduction efforts.
Hence, while selecting a predicate equipment, make sure that it has a strong safety history and is designed with the user's safety in mind. This proactive approach to equipment selection can minimize potential risks and contribute to the safety of patients and the public at large.
Devices Without Design-Related Recalls
Choosing healthcare instruments without a record of design-related recalls is a crucial aspect in guaranteeing patient safety and adherence to FDA regulations. The lack of recalls can be a sign of a product's reliability and adherence to thorough testing and quality standards. For example, the Philips Respironics case highlights the importance of thorough evaluation. Their machines, which lacked extensive human testing before market release, were associated with over 100,000 reports of injuries and even deaths, culminating in a discontinuation of sales and severe regulatory scrutiny.
Nonconformance with specified requirements, whether due to supplier changes, manufacturing errors, design defects, or human error, can lead to serious consequences. As noted by a former FDA analyst, such nonconformance, especially when not properly documented and addressed, poses high risks including regulatory penalties and recalls. This underscores the necessity of a robust quality management system that employs data-driven approaches rather than manual, document-based methods, which are prone to error. Leveraging digital continuity and artificial intelligence for quality trend analysis can significantly reduce the incidence of nonconformance.
Considering these factors, instruments that have effectively navigated the intricate terrain of regulations for healthcare equipment without recalls demonstrate a demonstrated history of safety and quality, rendering them preferable options in clinical environments.
How to Find and Use Predicate Devices
The recognition and application of related mechanisms is an essential element of the medical tool development procedure, especially when navigating the regulatory environment for FDA approvals. It requires a systematic approach to ensure that the chosen element is legally marketed and possesses the same intended use without raising new questions of safety and effectiveness. As part of the FDA's 510(k) modernization initiative, a currently registered product with the agency must be utilized. When exploring the intricacies of analogous equipment, it is crucial to take into account the technological attributes and the long-term safety information that may come with older equivalents.
The FDA has recently emphasized the development of best practices for predicate selection, releasing a draft guidance document on September 7, 2023, which aims to streamline the 510(k) submission process. This guidance is part of an effort to enhance efficiency and maintain the rigorous standards necessary for market approval. As the healthcare industry continues to evolve, with a substantial number of independent practices being acquired by larger entities in 2021, the reliance on advanced technologies and digitalization becomes increasingly important. This shift highlights the importance for developers of healthcare equipment to possess a comprehensive comprehension of the regulatory prerequisites and market dynamics.
Additionally, the market for healthcare equipment, encompassing a variety of diagnostic, therapeutic, and monitoring tools, plays a crucial part in patient care. The pricing of healthcare equipment is impacted by a variety of elements including creation expenses, rival producers, and product life cycle. Companies often adjust their pricing strategies based on internal policies and sales targets, with geographical location and facility type also impacting the final selling price. Understanding the market dynamics is crucial for medical instrument developers to navigate the industry effectively.
Searching the FDA 510(k) Database
The FDA’s 510(k) Premarket Notification database serves as a critical tool for manufacturers to identify precedent devices, enabling them to navigate the regulatory landscape with greater precision. To make the most of this resource, it's crucial to acquire a thorough understanding of the apparatus, encompassing its intended purpose and technological attributes. Through careful examination of the database using particular criteria, manufacturers can match their offerings with products that have been previously approved, thus simplifying the process for FDA compliance.
To guarantee the successful traversal of the 510(k) pathway, the first step entails verifying that a potential benchmark is a lawfully marketed product and presently registered with the FDA. After this, an assessment is needed to determine if the equipment shares the same intended use and whether any differences in technology do not introduce new safety and efficacy concerns. It is paramount to create a comparative analysis that includes a deep dive into the competitive environment, examining various materials such as clinical studies, marketing literature, and FDA guidance documents.
While the FDA continues its efforts to update and improve, it gives importance to choosing references that have been approved through established procedures, which may involve acknowledged consensus criteria or qualified tools for developing healthcare products. In some cases, well-accepted scientific methods that have undergone public scrutiny may also be appropriate. The agency's commitment to improving the 510(k) process was further highlighted in its recent draft guidance released on September 7, 2023, outlining best practices for selection of previous products.
In the end, the careful utilization of the FDA's 510(k) database and compliance with the agency's optimal procedure suggestions are crucial in guaranteeing that healthcare equipment not only fulfills regulatory requirements but also supports the FDA's goal of safeguarding public well-being by ensuring the safety, efficiency, and protection of healthcare technology.
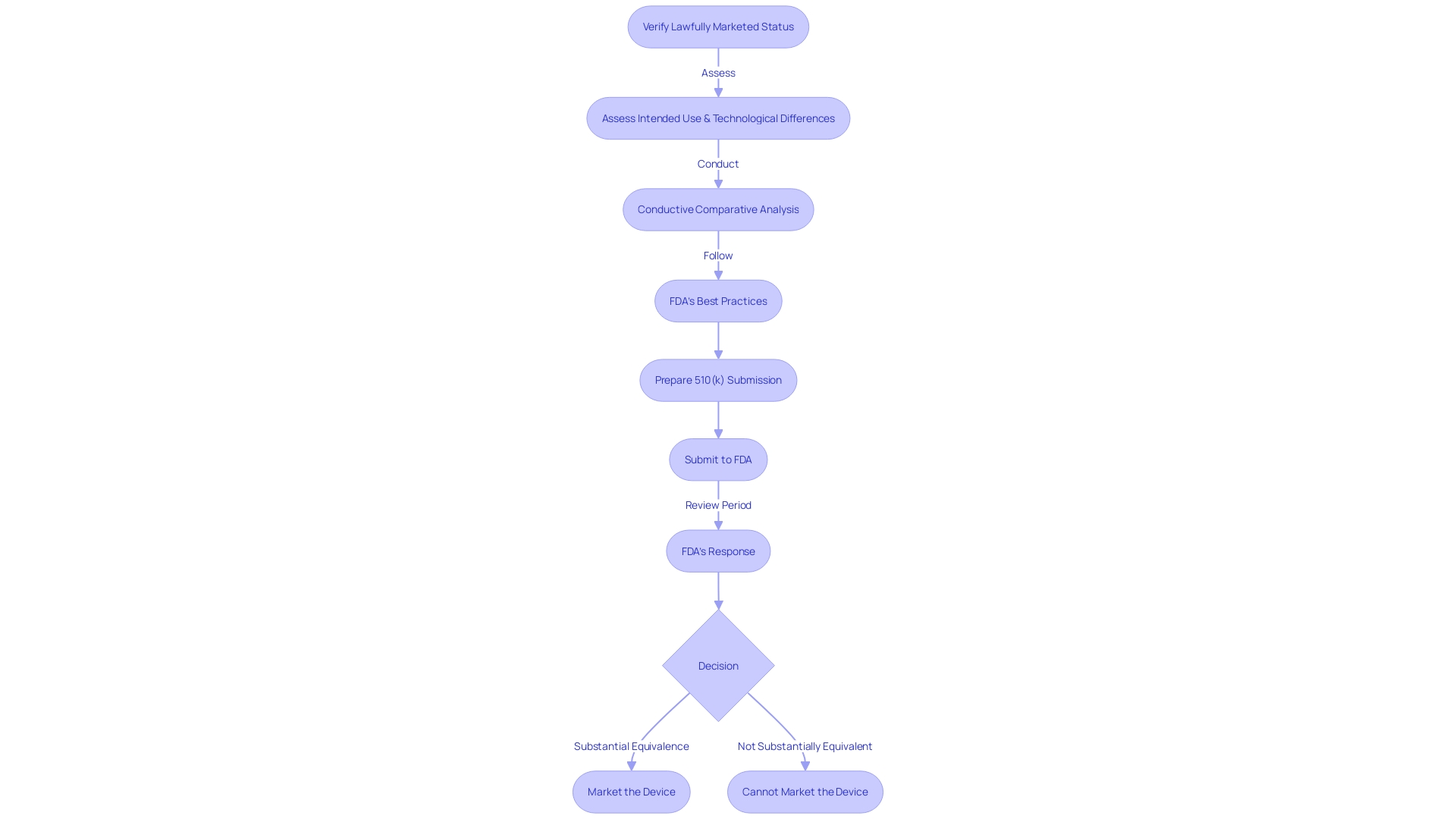
Identifying Valid Predicate Devices
Recognizing an antecedent apparatus is an essential phase in the FDA's 510(k) clearance procedure for medical instruments. It involves confirming that the potential product is a legally marketed item, presently registered with the FDA, and ensuring that it has the same intended purpose without introducing new concerns about safety and effectiveness due to differing technological features. The recent FDA draft guidance on the selection of previous examples as best practices, released on September 7, 2023, emphasizes the importance of this evaluation. This undertaking follows historical examination, as emphasized by the 2018 documentary 'The Bleeding Edge,' which uncovered the potential risks associated with apparatuses cleared without necessitating clinical trials. Such risks have included severe patient injuries, prompting a re-evaluation of the process. The FDA's responsibility in safeguarding public well-being by ensuring the safety and efficacy of healthcare instruments is of utmost importance, and these guidelines are a component of their continuous endeavors to improve the evaluation procedure, even for instruments with long-term safety information from previous models. As professionals in the industry, it is crucial to comprehend the intricacies of selecting the base instrument. This understanding is necessary for adhering to regulations and ensuring patient well-being. It also positions medical device development companies for favorable business outcomes such as acquisitions, IPOs, or strategic alliances.
Documenting Substantial Equivalence
To establish substantial equivalence when using a reference product for comparison, it is imperative to meticulously document the similarities between the new product and the reference. This documentation must cover a thorough examination of both gadgets' technological characteristics, intended use, and performance data. As observed in the case of industry leaders such as Medtronic plc, which provides a range of technologies addressing different health conditions, it is evident that each product must be thoroughly assessed based on its qualities. Thorough conformity assessment, as defined by the Office of Management and Budget (OMB), is crucial in demonstrating that a new equipment meets the stringent requirements set forth by the FDA. The FDA upholds the confidence of the public by guaranteeing the safety, effectiveness, and security of medical equipment, which is why such comprehensive documentation is not only a regulatory prerequisite but also a crucial measure in preserving the integrity of the medical equipment market. Additionally, the adherence to voluntary consensus standards and the implementation of rigorous conformity assessment protocols are instrumental in fostering innovation and standardization within the industry, ultimately enhancing patient access to novel devices.
Conclusion
In conclusion, selecting the right predicate device is crucial for the successful development and approval of new medical devices. The FDA's 510(k) modernization efforts stress the importance of choosing predicates that are legally marketed, have the same intended use, and don't raise new safety concerns.
Transparent communication about a device's purpose, development, performance, and logic is vital for healthcare professionals and patients to understand and trust it. Incorporating established methodologies and user-centered design principles ensures reliability and consistency throughout the evaluation process.
Safety and effectiveness are paramount in the medical device industry. Predicates should align with regulatory standards and undergo post-market surveillance. The FDA's commitment to public health is evident in its rigorous approval processes and reliance on voluntary consensus standards.
Identifying and using predicate devices require a systematic approach. The FDA's 510(k) database is a valuable resource for manufacturers to find predicates that have been cleared. Careful analysis of the database and adherence to the FDA's guidance on predicate selection streamline the submission process and ensure compliance with regulatory standards.
In conclusion, selecting the right predicate device is critical for the development and approval of medical devices. It requires careful evaluation, adherence to regulatory standards, and transparent communication. By following these best practices, manufacturers can ensure regulatory compliance, patient safety, and successful market entry.




