Introduction
Premarket Approval (PMA) represents a critical regulatory pathway established by the U.S. Food and Drug Administration (FDA) to ensure the safety and efficacy of Class III medical devices, which are often high-risk and complex. Unlike the 510(k) process, which is applicable for devices substantially equivalent to already marketed ones, PMA is mandatory for devices without a predicate device, necessitating a thorough review of clinical data, manufacturing protocols, and product labeling. This meticulous process underscores the FDA’s commitment to public health by ensuring only the highest standard devices make it to market.
From cardiovascular catheters to other essential medical tools, the PMA process rigorously evaluates each device's safety and performance, making it indispensable for advancing medical innovations. As the regulatory landscape evolves, understanding the nuances of PMA versus 510(k), the components of a PMA application, and best practices for submission is crucial for manufacturers aiming to navigate this complex framework and bring their products to market successfully.
What is Premarket Approval (PMA)?
Premarket Approval (PMA) is a stringent regulatory framework established by the U.S. Food and Drug Administration (FDA) for evaluating the safety and efficacy of Class III medical products, which are typically high-risk. Unlike the 510(k) process, which applies to products deemed substantially equivalent to pre-existing ones, PMA is mandatory for items that pose a significant risk to patient health. This rigorous process entails a comprehensive review of clinical data, manufacturing practices, and product labeling to ensure compliance with FDA standards.
The PMA process is crucial for products where no predicate product exists, making it indispensable for innovations in the medical field. According to FDA guidelines, the decision to recognize or not recognize a consensus standard follows within 60 calendar days from the date the request is received. Upon decision, the FDA updates its Recognized Consensus Standards Database with a Supplemental Information Sheet (SIS) that details the rationale behind the decision.
FDA's thorough method in the PMA process is highlighted by the necessity to maintain public health and well-being. This procedure guarantees that only items meeting the highest standards are authorized for market entry. As per the FDA, “Using the wrong terminology can impact your company’s reputation, and possibly have some legal implications, but more importantly, it can mean that you don’t have a clear understanding of how to bring your product to market.”
Moreover, the FDA's Center for Drug Evaluation and Research (CDER) provides clarity on study design elements and data requirements necessary for a thorough assessment of new drugs and therapeutic products. This dedication to scientific rigor and patient safety is reflected in the PMA process, ensuring that the most critical products undergo the most exhaustive review procedures.
Why is PMA Required?
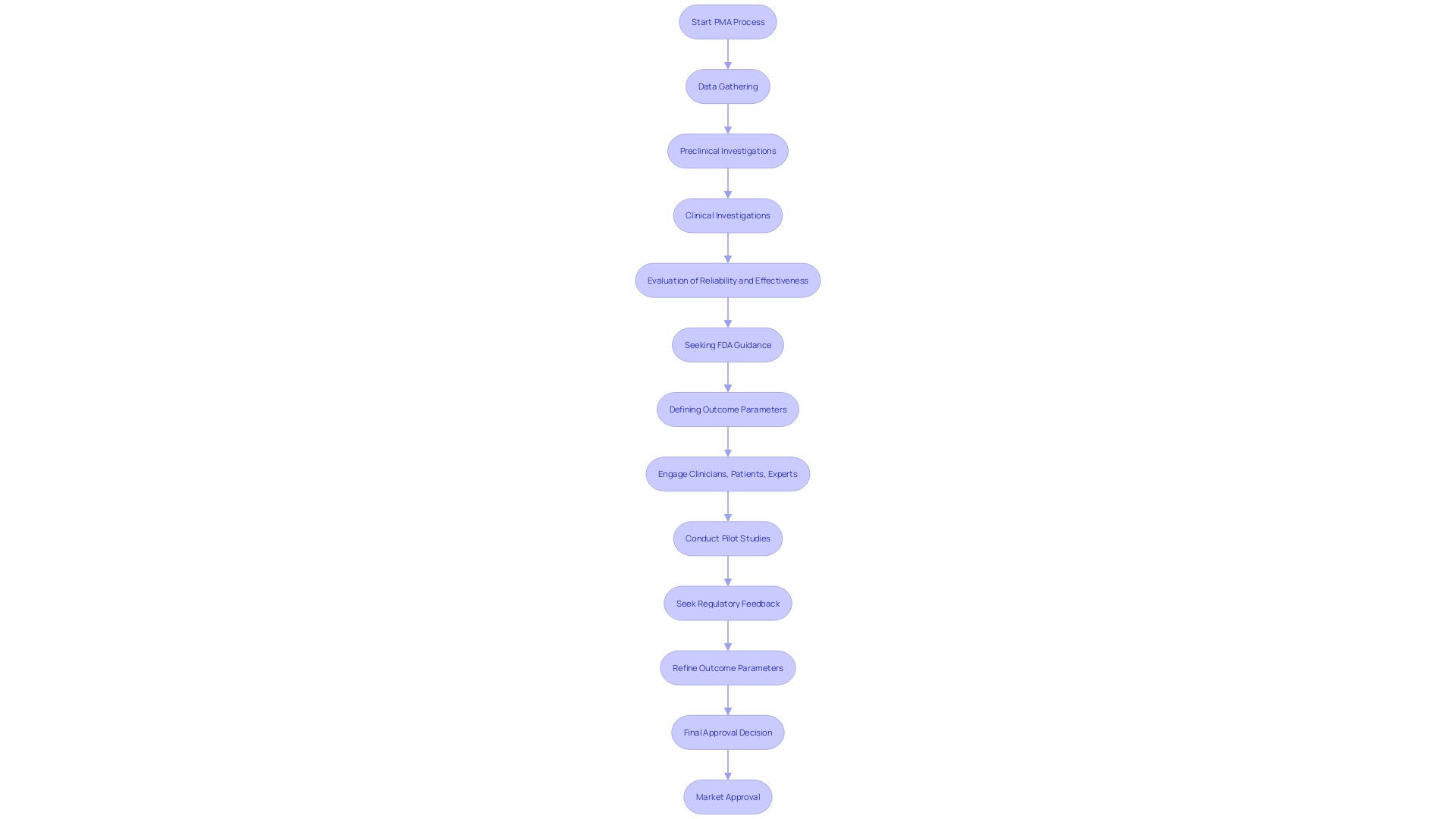
Premarket Approval (PMA) is essential for Class III products due to their inherent higher risks and complexities. These instruments, such as cardiovascular catheters and other essential medical tools, require rigorous scrutiny to ensure they are safe and effective before entering the market. The PMA procedure includes a detailed review by the FDA, which evaluates the product's reliability and effectiveness through extensive data gathering and examination. This stringent regulatory requirement helps safeguard public health by ensuring that only products meeting the highest standards of quality and safety are available for use. Moreover, the FDA's classification system, which encompasses over 1,700 unique categories of items, assists manufacturers in identifying the suitable risk class and regulatory route for their products, guaranteeing adherence to crucial quality system standards and post-market monitoring protocols.
PMA vs 510(k): Key Differences
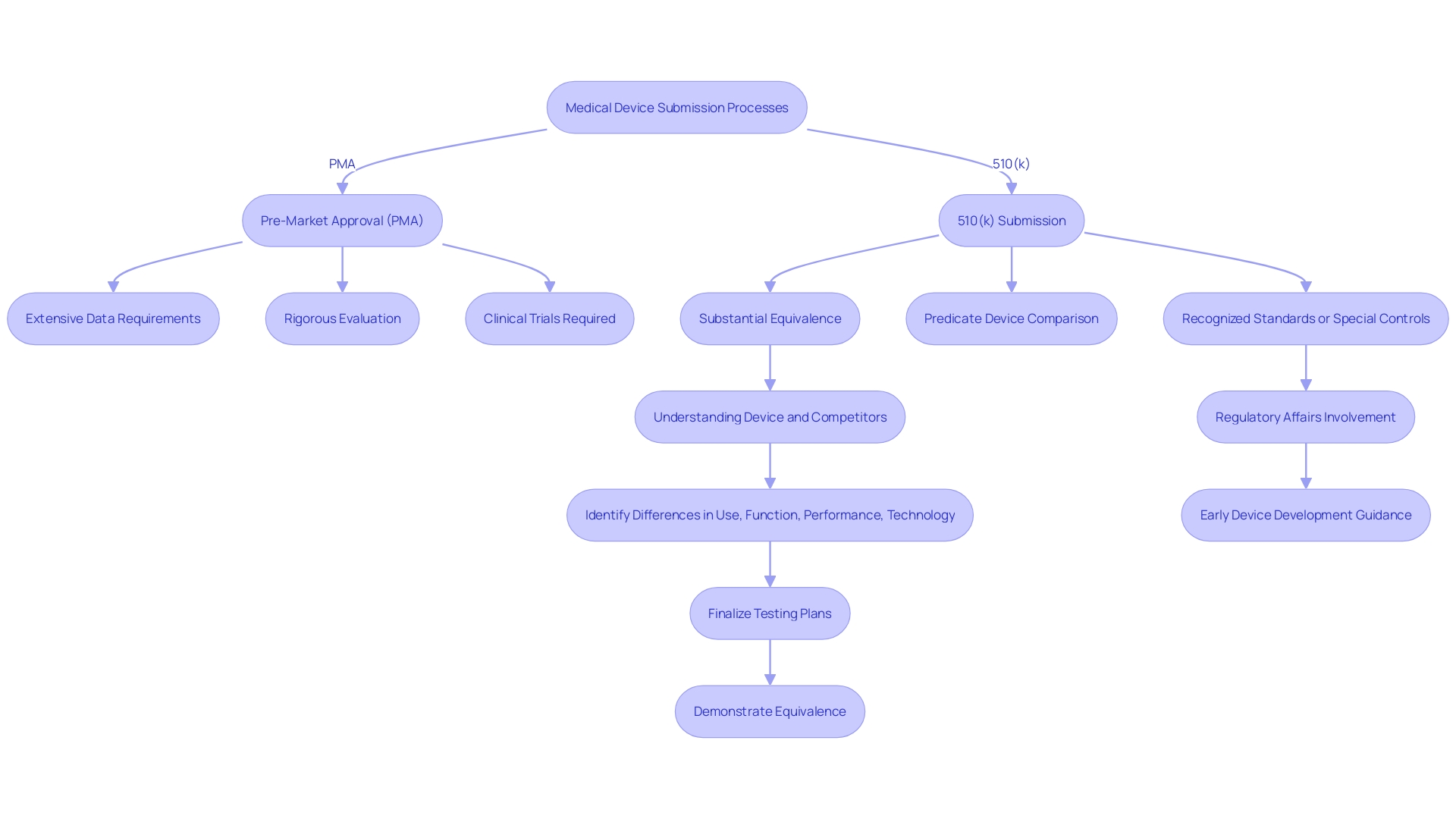
The PMA process is markedly distinct from the 510(k) submission pathway. The 510(k) submission aims to demonstrate that an instrument is substantially equivalent to a legally marketed item, making it a less rigorous process that often involves compiling existing data and providing comparative studies. In contrast, the PMA process requires extensive scientific evidence and comprehensive clinical data to substantiate claims of security and effectiveness. This pathway is more intricate, typically requiring clinical trials and detailed documentation to meet the FDA’s stringent requirements.
The PMA process is inherently lengthier and more involved. It requires a thorough evaluation of the equipment's performance through clinical trials, which can be time-consuming and resource-intensive. Moreover, unlike the 510(k) pathway, which may rely on existing data, PMA applications necessitate new, original research and data collection, often involving multiple phases of clinical trials. This thorough examination guarantees that only products with demonstrated reliability and effectiveness enter the market, thereby offering a greater degree of confidence for both patients and healthcare professionals.
Additionally, the PMA process includes post-market surveillance (PMS) to continuously monitor the product’s performance in real-world settings. Various methods, such as passive surveillance systems, active registries, and electronic health records, are employed to collect valuable data. This continuous observation aids in recognizing and reducing possible hazards, guaranteeing the long-term security and efficacy of the medical instrument.
In summary, while the 510(k) pathway offers a quicker and less burdensome route to market, the PMA process, with its rigorous requirements and comprehensive evaluation, provides a more robust assurance of a product’s safety and effectiveness. Comprehending these distinctions is essential for medical equipment firms maneuvering through the regulatory environment.
Components of a PMA Application
A complete PMA application consists of several key components, including:
- Device Description: Detailed information about the device, including its design and intended use.
- Manufacturing Information: Data on the manufacturing processes, quality control measures, and facilities involved in production. Maintaining complete visibility into the Bill of Materials (BOM), including component serialization and lot traceability, is crucial for risk management.
- Clinical Data: Results from clinical trials that demonstrate the safety and effectiveness of the apparatus. This may include data from passive surveillance systems, such as spontaneous reporting by healthcare professionals and patients, and active surveillance through registries or studies.
- Labeling: Proposed labeling that includes instructions for use, indications, and contraindications. The FDA emphasizes the use of consumer-friendly language in prescription drug advertisements to ensure clarity and comprehensibility.
- Post-Market Surveillance Plan: A strategy for monitoring the product's performance once it is on the market. Post-market surveillance (PMS) employs various methods, including the utilization of electronic health records and administrative databases, to continuously monitor products in real-world settings. This stage is essential for recognizing and tackling possible security concerns and enhancing equipment performance over time. 'The importance of PMS cannot be exaggerated, as it plays a crucial role in patient protection by identifying and reducing potential hazards linked to medical instruments.'.
PMA Application Process
The PMA application procedure involves multiple essential phases, each intended to guarantee the security and effectiveness of medical products prior to their arrival in the marketplace.
-
Pre-Submission: This initial phase involves engaging with the FDA to discuss study design and data requirements. According to Dr. John Sheets, former head of the FDA’s Office of Device Evaluation, these interactive meetings are pivotal, as they provide an opportunity to gather feedback before the planned premarket submission.
-
Submission: At this stage, the PMA application is completed and submitted to the FDA. This thorough document contains all essential information to back the security and efficacy of the apparatus.
-
FDA Review: An in-depth review is conducted by FDA staff. This rigorous process often involves requests for additional information to ensure all aspects of the item's performance are thoroughly evaluated. The FDA’s draft guidance on the Q-Submission Program highlights the importance of clear communication during this phase to address any queries effectively.
-
Advisory Committee Review: An independent advisory panel may be convened to provide expert guidance on the application. This step ensures that the evaluation process benefits from diverse expertise and perspectives.
-
FDA Decision: Finally, the FDA makes a decision to approve the PMA, request further information, or deny the application. This decision is based on a comprehensive assessment of the data submitted and the advisory committee’s recommendations.
The complete PMA procedure is intended to maintain the utmost levels of patient protection and product effectiveness, showcasing the FDA’s dedication to community health.
PMA Review Timeline and Expectations
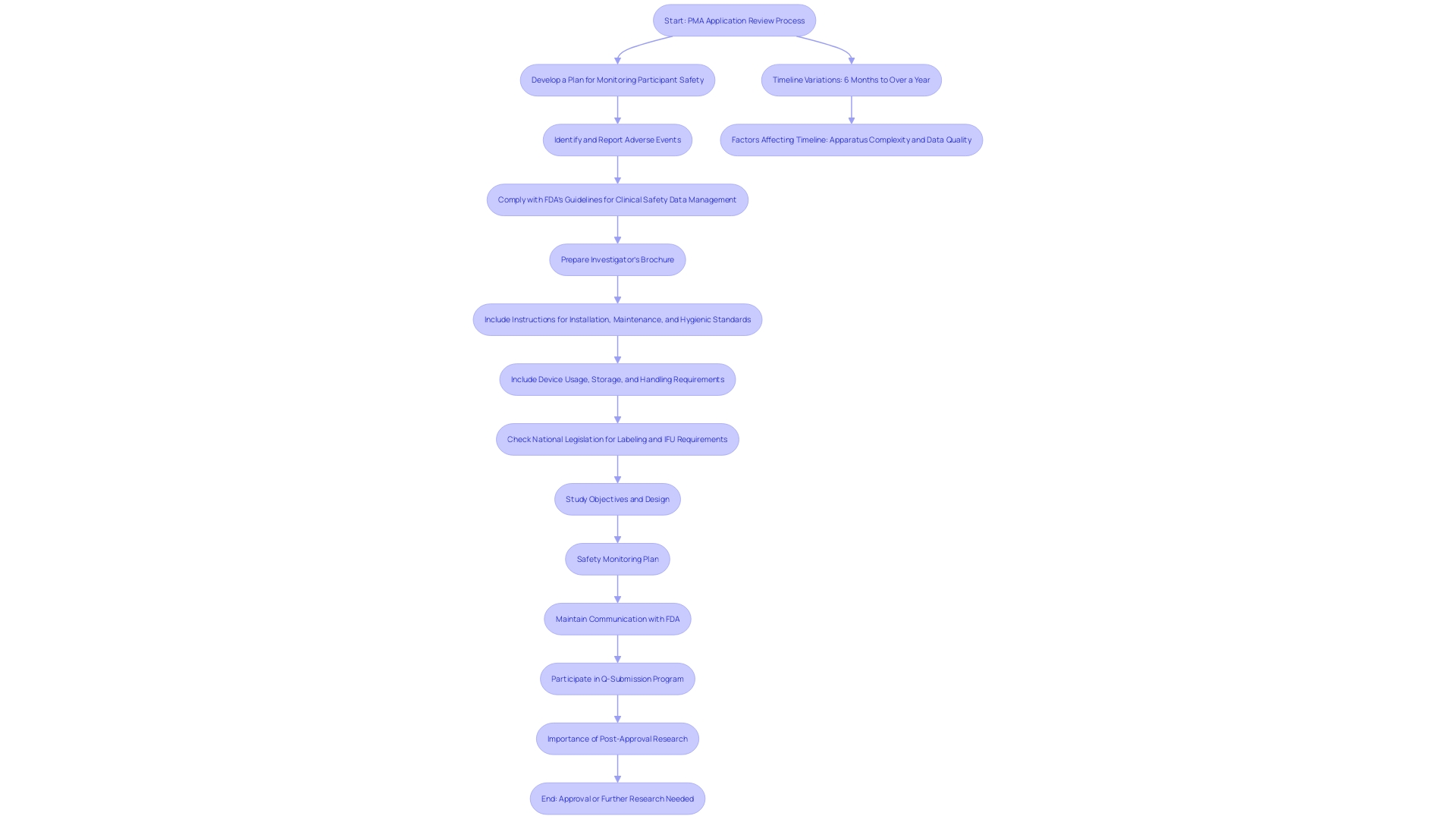
The review timeline for a PMA application can vary significantly, generally ranging from six months to a year or more. Factors impacting this timeline include the complexity of the apparatus, the quality of the submitted data, and the FDA's workload. The FDA has been proactively tackling issues in its monitoring systems, aiming to improve equipment protection oversight. With more than 1.7 million injuries and 83,000 fatalities associated with medical apparatus over ten years, the significance of strong monitoring cannot be emphasized enough. Applicants should prepare for potential delays and maintain open communication with the FDA throughout the review process. Engaging in the Q-Submission Program offers opportunities to clarify requirements and streamline the review. Furthermore, post-approval research frequently required by the FDA is essential for guaranteeing long-term well-being and effectiveness, necessitating regular reporting and potential PMA supplements for any major alterations.
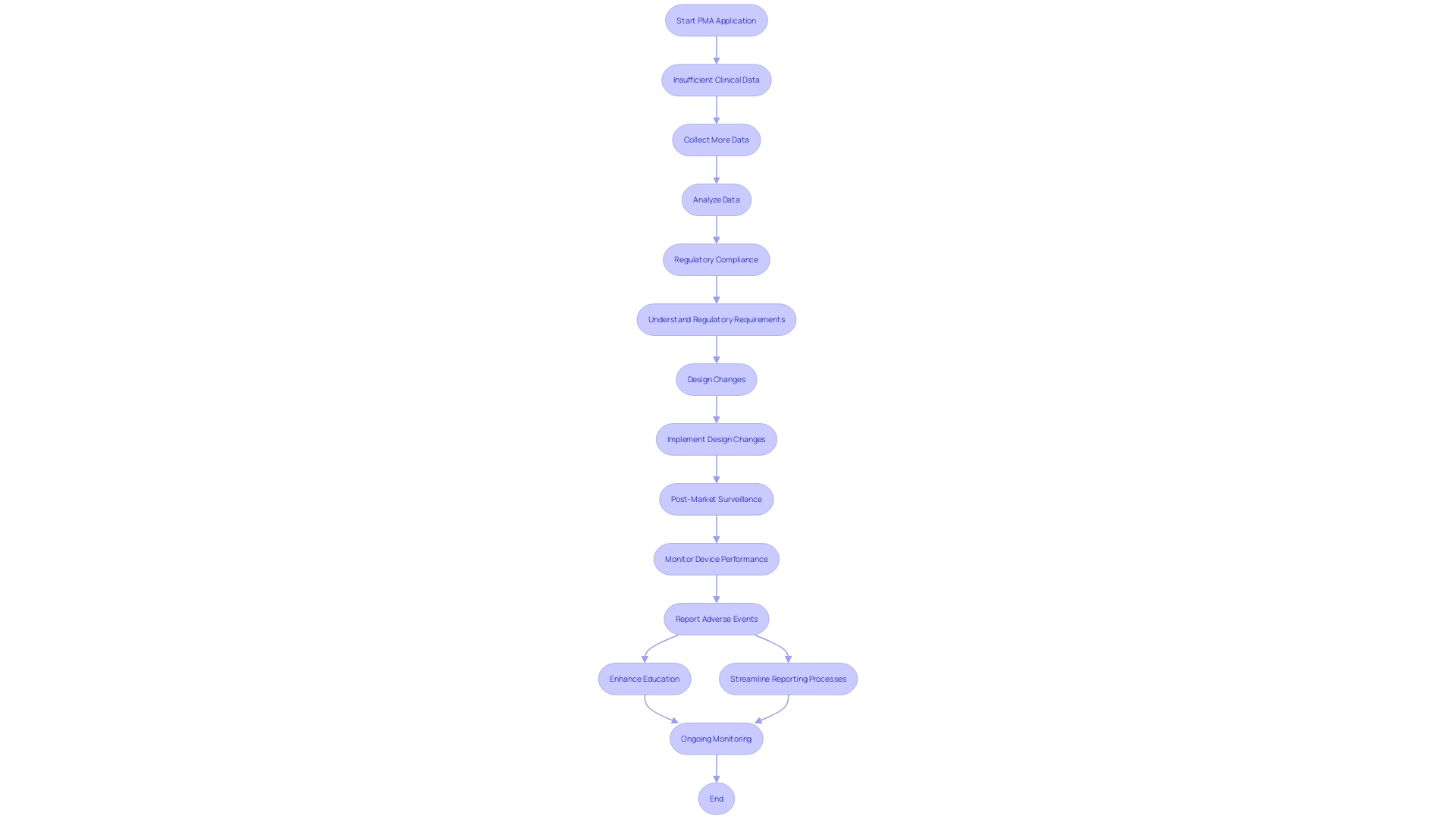
Common Challenges in PMA Applications
PMA applications often encounter several significant challenges:
-
Insufficient Clinical Data: One of the primary hurdles is providing robust clinical evidence to substantiate claims of safety and effectiveness. This can be particularly daunting as it requires comprehensive data collection and rigorous analysis to meet the FDA's stringent standards.
-
Regulatory Compliance: Navigating the complex web of FDA requirements demands meticulous attention to detail. The regulatory landscape is constantly evolving, and staying abreast of these changes is crucial. The FDA's updated guidance on the use of real-world evidence underscores the importance of high-quality data in regulatory decision-making, adding another layer of complexity.
-
Changes in Design: Modifications to the equipment during the review process can necessitate additional submissions or data. 'These adjustments must be carefully documented and justified to ensure they do not compromise the equipment's safety or effectiveness.'. For instance, any changes affecting the performance of the equipment must be submitted as a PMA supplement before implementation.
Addressing these challenges requires a thorough understanding of the regulatory framework and a proactive approach to data collection and compliance. Ongoing post-market surveillance (PMS) plays a crucial role in this process, allowing for the identification and reduction of potential risks linked to medical instruments. Through approaches like passive monitoring, active records, and the use of electronic health documentation, PMS offers important insights into the long-term reliability and efficacy of products, thus aiding continuous regulatory adherence and patient well-being.
Best Practices for Successful PMA Submissions
To enhance the chances of a successful PMA submission, consider these best practices:
-
Engage with the FDA Early: Utilize pre-submission meetings to clarify expectations and requirements. Early engagement facilitates a clear understanding of the regulatory pathway and helps address potential issues before submission. The FDA encourages sponsors to comment on draft guidance documents before finalization to ensure their input is considered.
-
Invest in Quality Clinical Trials: Ensure that clinical trials are well-designed, adequately powered, and compliant with regulatory standards. As per FDA guidelines, the outcomes of clinical investigations involving human participants, including study protocols, effectiveness data, and adverse reactions, are essential for PMA approval. As Mike Drues from the Global Medical Device Podcast highlights, “just because you're meeting the standard, that doesn't necessarily mean that you're making a safe and effective product.” Thus, rigorous clinical data collection is imperative.
-
Thorough Documentation: Maintain detailed documentation throughout the development process to support the PMA application. This encompasses detailed documentation of study advancement, security and effectiveness information, and any modifications to the apparatus. For example, producers carrying out post-approval research must present regular reports outlining study advancement and results to guarantee ongoing reliability and effectiveness.
These practices not only streamline the PMA process but also contribute to the long-term safety and performance of medical devices.
Conclusion
The Premarket Approval (PMA) process is a vital regulatory pathway that ensures the safety and efficacy of Class III medical devices, which are often associated with higher risks. This rigorous framework stands in contrast to the 510(k) process, demanding comprehensive clinical data and a thorough review of manufacturing protocols. The meticulous nature of PMA underscores the FDA's commitment to public health, ensuring that only devices meeting the highest standards are approved for market entry.
Understanding the distinctions between PMA and 510(k) is essential for manufacturers. While the 510(k) pathway may offer a quicker route to market through equivalency claims, the PMA process provides a more robust assurance of safety and effectiveness through extensive clinical trials and continuous post-market surveillance. This ongoing monitoring is crucial for identifying potential risks and enhancing patient safety in real-world settings.
To navigate the complexities of the PMA application process successfully, manufacturers are encouraged to engage early with the FDA, invest in high-quality clinical trials, and maintain thorough documentation. These best practices not only facilitate a smoother submission process but also contribute to the long-term success and reliability of medical devices. By adhering to these guidelines, manufacturers can better align their products with regulatory expectations, ultimately advancing innovations that prioritize patient safety and public health.




