Introduction
In the complex landscape of medical device regulation, the 510(k) submission process stands as a critical pathway for manufacturers seeking to bring their devices to the U.S. market. This regulatory requirement applies broadly, encompassing startups, established firms, and international manufacturers. Understanding whether a device necessitates a 510(k) submission involves a thorough examination of its classification and intended use.
Manufacturers must meticulously educate themselves on the device, its users, and its competitive environment. This includes analyzing clinical studies and identifying potential predicate devices to establish substantial equivalence—a key determinant of successful submissions. Given that a significant portion of 510(k) submissions face initial rejection due to issues with substantial equivalence, comprehensive preparation is paramount.
Device classification further complicates this process, as medical devices are categorized into Class I, Class II, and Class III based on their risk levels, each with different regulatory requirements. Identifying a suitable predicate device, ensuring accurate device classification, and preparing a detailed and organized submission are essential steps to navigating this regulatory pathway effectively. Awareness of common pitfalls and proactive planning can significantly enhance the likelihood of approval, facilitating the safe and effective introduction of new medical devices to the market.
Who Needs to Submit a 510(k)?
The 510(k) application process is crucial for producers seeking to promote a medical product that is not exempt from premarket notification requirements. 'This pertains to a wide range of stakeholders, including startups, established manufacturers, and international entities intending to sell products in the U.S. To ascertain whether their product requires a 510(k) submission, it's essential for these parties to comprehend the classification and intended use of the product.'.
'Learning about the topic and its purpose is essential.'. This involves gaining a deep understanding of the users of the system—such as clinicians, physicians, and patients—and its instructions for use, including any warnings and cautions. Working together with marketing groups to understand the competitive environment and concentrate on rival products is also advantageous. Examining research literature, clinical studies, and other pertinent materials can assist in recognizing possible predicate items with the same intended use and comparable technological traits, aiding in the development of a comparative table.
Grasping significant equivalence is another crucial aspect. According to the FDA, an instrument is substantially equivalent to a predicate instrument if it has the same intended use and either the same technological characteristics or different technological characteristics that do not raise new questions of safety and effectiveness. This idea is crucial, as 75% of 510(k) applications are denied on the initial attempt, with 85% of those denials caused by problems related to equivalence during the scientific evaluation.
For example, Boston Scientific was the first company to obtain a 510(k) clearance in 2000, highlighting the significance of substantial equivalence to a predicate product. This process is a cornerstone of the 510(k) submission, and thorough preparation can significantly enhance the likelihood of a successful application.
Understanding Device Classification and the 510(k) Pathway
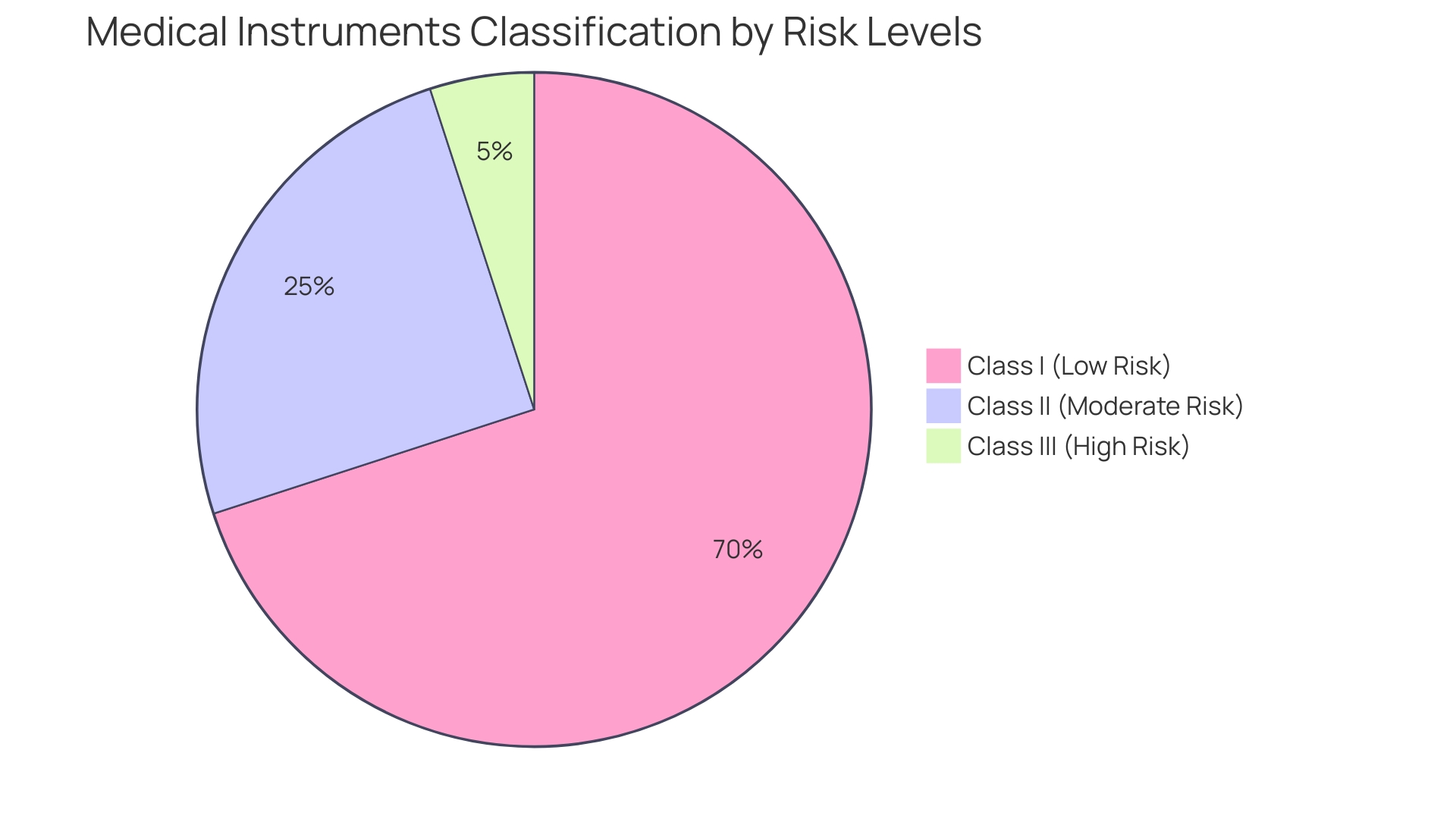
'Medical instruments are categorized into Class I, Class II, and Class III based on their risk levels to patients.'. Class I items present the lowest risk and necessitate minimal regulatory oversight. Class II products, which account for a considerable share of the market, must show substantial equivalence to a predicate item already available through the 510(k) pathway. Class III products, carrying the highest risk, necessitate Premarket Approval (PMA) due to their potential impact on patient safety. Before promoting any product in the US, manufacturers must determine the correct classification and select the suitable regulatory pathway, whether it be 510(k), PMA, or the De Novo process. The FDA offers an extensive classification database, encompassing over 1,700 categories across different medical specialties, to assist in this determination. Ensuring the right classification is crucial for compliance and successful market entry.
What is a 510(k) Predicate Device?
A predicate product is a legally marketed item that serves as a benchmark for comparison in a 510(k) submission. Identifying a suitable predicate tool requires manufacturers to ensure it has the same intended use and similar technological characteristics as the new item. This requires a profound comprehension of the subject equipment, its purpose, and the competitive environment, including current products available in the market. As per the FDA's definition, significant equivalence implies that the new apparatus must either possess the same technological traits as the predicate or, if dissimilar, must be demonstrated to be as safe and effective without introducing new safety issues. 'Establishing substantial equivalence is crucial as it demonstrates the new product's safety and efficacy, ultimately facilitating its market entry.'.
Key Components of a 510(k) Submission
A thorough 510(k) application includes several essential components, such as a detailed product description, proposed labeling, and extensive information on the predicate item. It is essential to provide data supporting the safety and effectiveness of the new equipment, which may include performance testing results, biocompatibility assessments, and, if applicable, clinical data. A well-structured application is essential for a successful evaluation by the FDA, as it shows significant equivalence to a legally marketed device. This is especially significant considering that 75% of 510(k) applications are initially denied, with 85% of those denials arising from concerns related to equivalence during the scientific evaluation. Therefore, a thorough understanding of the competitive landscape and a detailed comparative analysis are fundamental for compliance and approval.
Common Pitfalls in 510(k) Submissions and How to Avoid Them
Navigating the 510(k) application process can be challenging, with common pitfalls that manufacturers should be aware of. These comprise poor description of the apparatus, insufficient information to back assertions of significant equivalence, and incomplete or vague labeling. Understanding the concept of substantial equivalence is crucial, as statistics indicate that 75% of 510(k) submissions are rejected at the first submission, with 85% of those rejections due to issues related to substantial equivalence.
To avoid these setbacks, it is essential to conduct thorough pre-submission planning. This involves educating yourself on the subject tool and its intended users, such as clinicians, physicians, and patients. Furthermore, explore the competitive environment by reviewing research literature, clinical studies, and rival products. Creating a comparative table of potential predicate devices with similar technological characteristics can be beneficial.
Seeking feedback from the FDA through pre-submission meetings is another critical step. These meetings provide an opportunity to clarify requirements and receive guidance on documentation. Ensuring all documentation is clear and comprehensive is vital to support claims and mitigate the risk of rejection.
For instance, the first 510(k) issued in 2000 went to Boston Scientific, highlighting the importance of meticulous preparation and understanding of the substantial equivalence criteria. By focusing on these areas, manufacturers can enhance their chances of a successful 510(k) submission.
Conclusion
The 510(k) submission process is essential for various stakeholders, including startups and international manufacturers, looking to market medical devices in the U.S. Determining the need for a 510(k) involves understanding the device's classification and intended use. A thorough grasp of the device, its users, and the competitive landscape is crucial, particularly in identifying predicate devices to establish substantial equivalence.
Medical devices are classified into Class I, Class II, and Class III based on risk, with Class II devices requiring demonstration of substantial equivalence to legally marketed predicates. The FDA's classification database serves as a key resource for proper classification.
Establishing substantial equivalence is critical for demonstrating safety and efficacy. A complete 510(k) submission must include detailed descriptions, proposed labeling, and supporting data. Given the high rejection rates of initial submissions, meticulous preparation is vital.
Common pitfalls include inadequate device characterization and insufficient data. To mitigate these risks, thorough pre-submission planning and seeking feedback from the FDA are advisable. By focusing on these areas, manufacturers can improve their chances of a successful 510(k) submission, paving the way for safe and effective market entry of medical devices.




