Introduction
The European Union has implemented the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR) to enhance the regulatory environment for medical devices. These regulations replace previous directives and establish updated requirements for market entry and compliance throughout a device's lifecycle. The MDR and IVDR address drug-device combinations, companion diagnostics, categorization and labeling of medical products, and post-market surveillance.
Manufacturers are currently adjusting their products to meet the stringent MDR requirements, emphasizing efficiency in regulatory documentation. The EU is also expediting the implementation of EUDAMED, a comprehensive database on medical devices. Industry reports highlight the impact of new regulations on device availability and strategies for maintaining compliance.
These changes demonstrate the EU's commitment to patient safety and innovation in the medical device sector.
Overview of the Directive on Medical Devices
The European Union has undertaken a notable revamp of its regulatory environment for healthcare equipment with the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR). These regulations override the previous directives, strengthening the legal framework for medical instruments, which encompass everything from simple bandages to life-saving medical equipment, ensuring they meet the latest standards for patient safety and effectiveness.
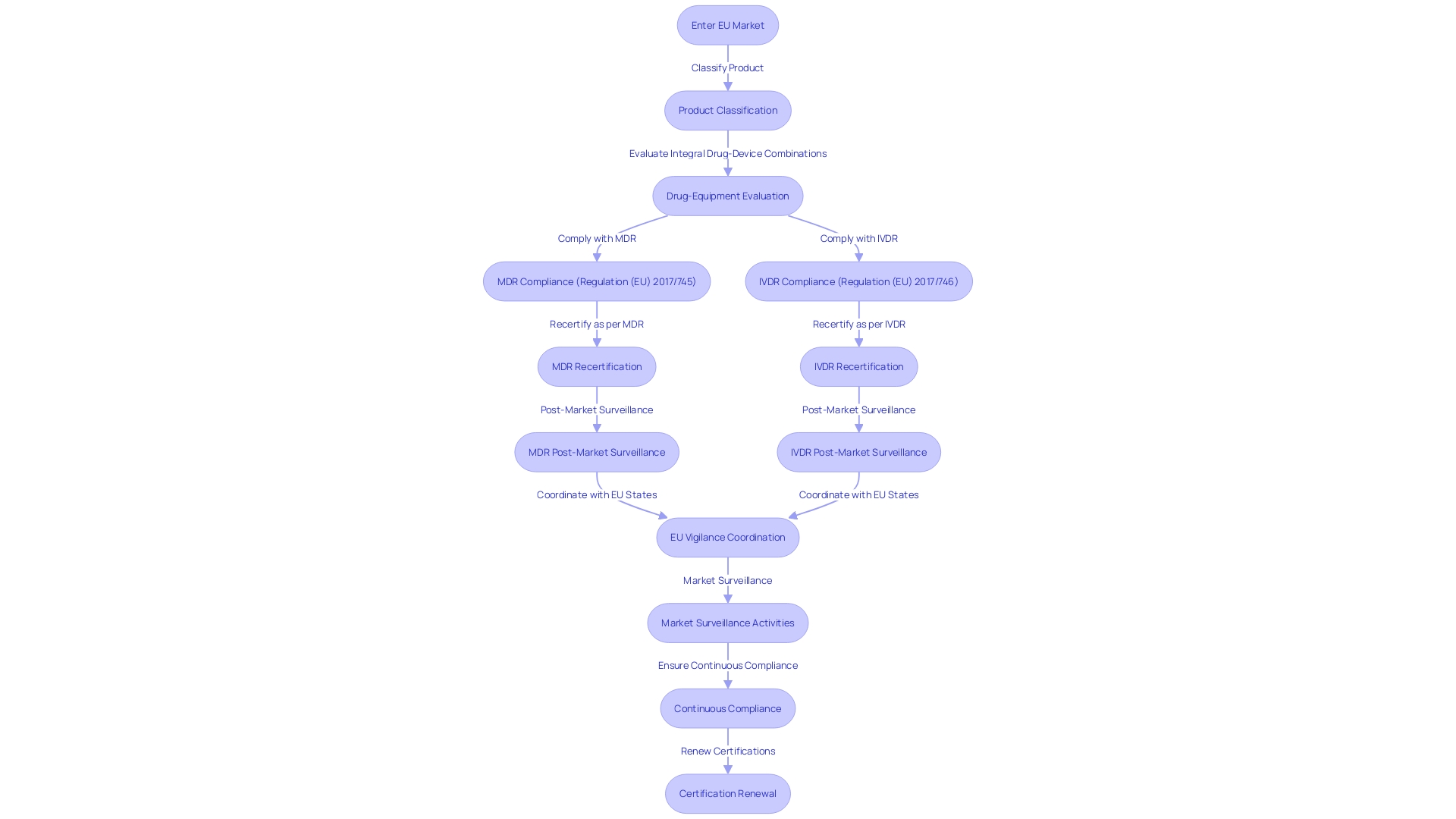
Under the MDR (Regulation (EU) 2017/745), manufacturers must carefully navigate updated requirements for market entry and maintain compliance throughout the product's lifecycle. The regulation outlines the responsibilities for the European Medicines Agency (EMA) and national competent authorities, especially in evaluating drug-equipment combinations, such as pre-filled syringes, and equipment with ancillary medicinal substances.
Similarly, the IVDR (Regulation (EU) 2017/746) has been implemented, which is especially relevant for diagnostic tools employed in conjunction with medicines. This includes companion diagnostics that are pivotal for the appropriate administration of specific treatments.
Significant revisions have been made based on accumulated experience and real-world incidents since the previous regulations were implemented. The fresh guidance deals with the classification and marking of therapeutic products that are packaged with instruments, advisory processes for instruments with medicinal substances, and the handling of essential drug-instrument combinations.
Manufacturers are currently in a transition phase, adjusting and recertifying their products to align with the MDR's stringent requirements. This includes an emphasis on post-market surveillance and enhanced coordination between EU member states for vigilance and market surveillance.
The EU's dedication to increased openness is apparent with the suggestion to accelerate the execution of EUDAMED, the European database on healthcare instruments. The database aims to provide a comprehensive picture of all medical products on the market by late 2025.
Recent reports reveal industry insights on preparedness for the new climate. There is an agreement on the increase of demands from regulations, the influence of new initiatives on the availability of devices, and approaches for upholding compliance in the midst of changing requirements. The industry is now prioritizing efficiency in regulatory documentation to minimize delays and errors, ensuring a smooth transition to the new regulations.
The reorganization of the European legal framework for healthcare tools, as indicated in these regulations, is a testament to the EU's dedication to safeguarding public health while supporting innovation within the medical equipment sector.
Scope and Definition of Medical Devices
Investigating the domain of healthcare instruments uncovers a variety of tools that are crucial in diagnosing, monitoring, and treating different health conditions. As per the World Health Organization, the definition of an instrument encompasses any tool, machine, appliance, software, material, or other article used alone or in combination, tailored for a variety of healthcare purposes. This includes everything from the common tongue depressor and disposable gloves to the intricately designed endoscopes and advanced prosthetic limbs. Notably, endoscopes represent a significant investment for healthcare facilities, with acquisition costs reaching up to $60,000, not including the substantial service contracts often necessary for their maintenance.
Moreover, the categorization of medical instruments by the FDA is a vital procedure that classifies instruments based on their intended purpose and level of regulation necessary to guarantee safety and effectiveness. The classification name, product code, and representative samples of advertisements and labeling material are all elements used to define and regulate these items. A 'restricted product' is one such category, denoting products that have sale, distribution, or use limitations as part of their regulatory status.
The healthcare equipment sector is a significant contributor to the healthcare market, with the United States expected to generate a revenue of around $215.80 billion in 2024. The market is expansive, encompassing not just diagnostic and therapeutic tools but also patient monitoring systems, surgical instruments, and dental equipment. This sector is marked by rapid growth due to ongoing innovation and a growing demand for advanced healthcare solutions. Therefore, instruments are essential tools in the quest for improved patient care and the enhancement of the quality of life for individuals dealing with different health conditions.
Classification of Medical Devices
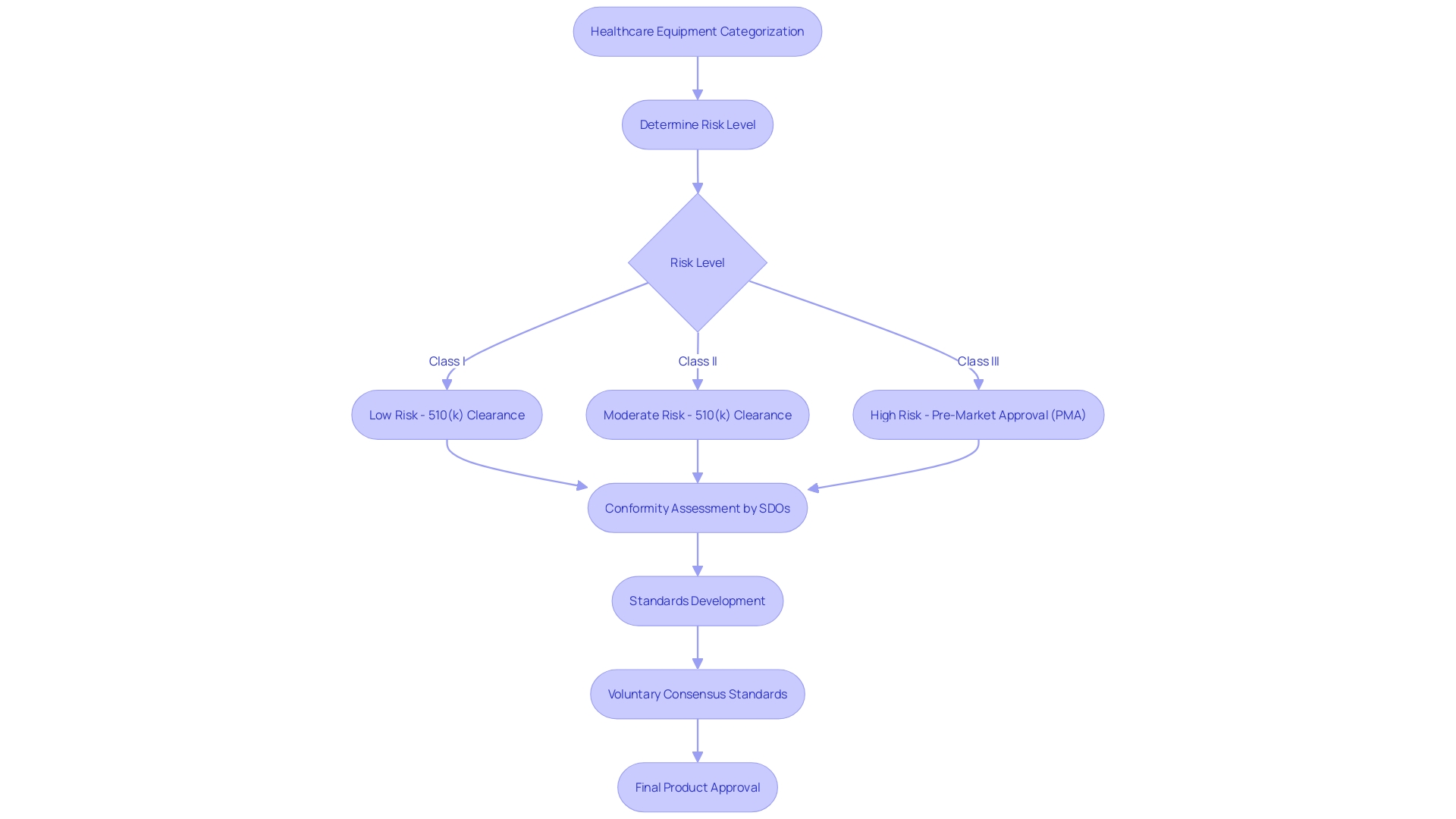
The categorization of healthcare equipment is a fundamental feature for manufacturers and governing entities alike, determining the extent of examination and assessment a product must undergo before it is available for sale. The U.S. Food and Drug Administration (FDA) categorizes medical apparatus into three classes based on the degree of risk they present to patients. Class I instruments, considered low-risk, and Class II instruments, which carry moderate risk, often follow a more simplified pathway, usually through a 510(k) clearance that compares the instrument to an already approved one. Meanwhile, Class III products are linked with high risk; they are vital for maintaining life or are implantable, such as pacemakers, and thus undergo a more extensive approval process, including Pre-Market Approval (PMA). Comprising roughly 10% of the instruments overseen by the FDA, these high-risk devices undergo thorough scrutiny to ensure the highest level of patient well-being and effectiveness.
To improve regulatory quality and ensure that equipment meets strict safety and effectiveness benchmarks, voluntary consensus standards are developed by Standards Development Organizations (SDOs). These standards are grounded in principles of transparency, stakeholder participation, balanced representation, and due process. The FDA, as part of its mandate to safeguard public health, utilizes these criteria in its conformity assessment, which is a thorough evaluation process encompassing testing, inspection, and certification to ensure that a healthcare equipment meets the necessary criteria.
Innovation and standardization in the field of healthcare equipment are driven by these agreement standards, promoting technological advancements that make it easier for patients to access new and improved medical devices. In an increasingly global market, harmonization of standards plays a critical role in maintaining a high bar for quality and security. Therefore, establishing the suitable equipment categorization is an initial but crucial phase; it influences the enrollment procedure and is essential to the journey of the healthcare instrument from its inception to practical application, a journey emphasized by the dedication to patient well-being and availability of healthcare technology.
Conformity Assessment Procedures
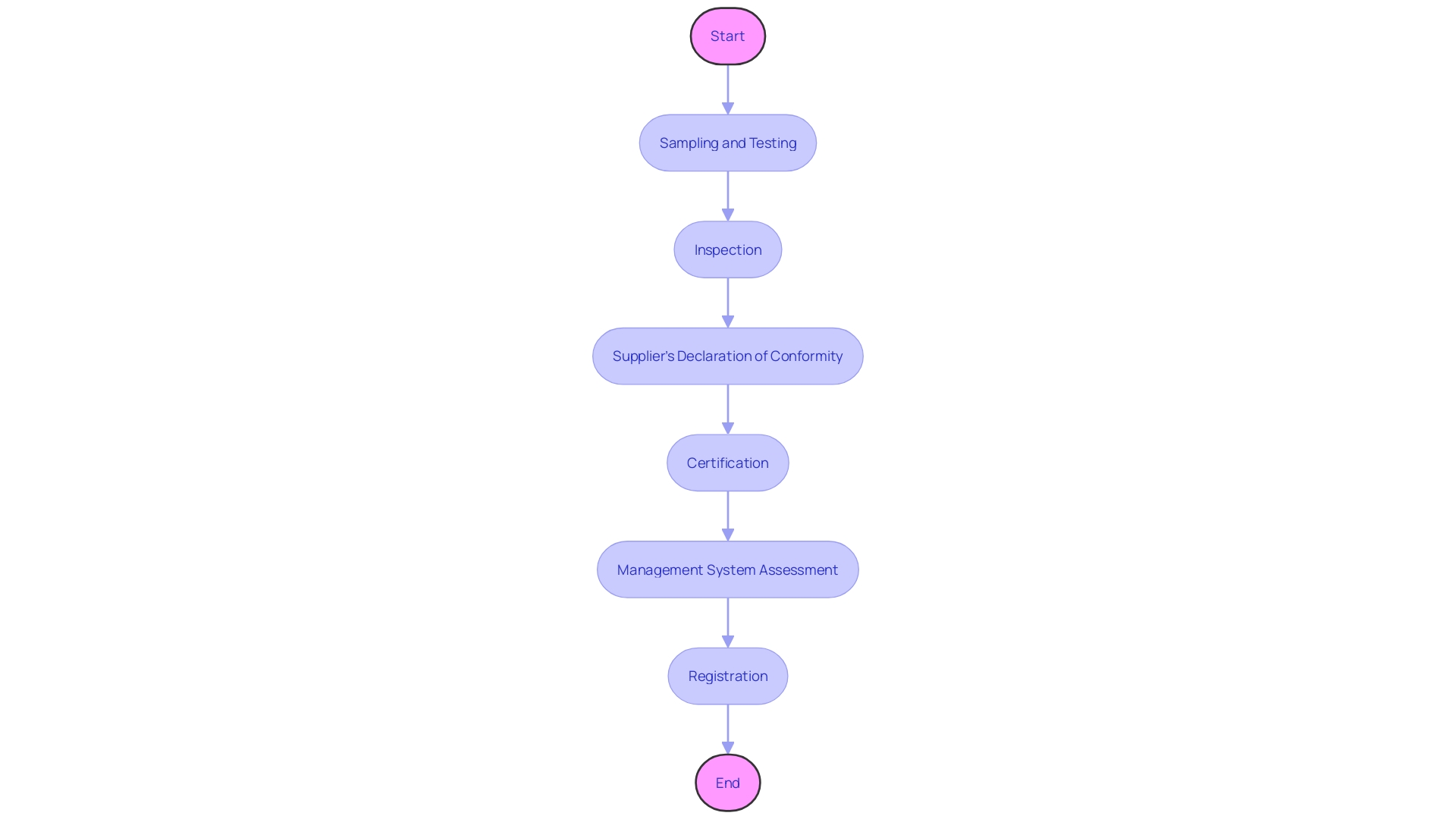
Conformity assessment procedures are the foundation of meeting the necessary requirements for devices, ensuring that they satisfy the required standards before reaching the market. These assessments are a systematic and comprehensive evaluation, encompassing various steps such as sampling and testing, inspection, supplier's declaration of conformity, certification, and management system assessment and registration. The use of voluntary consensus standards, developed by Standards Development Organizations (SDOs) both domestically and internationally, plays a crucial role in this process. These standards are crucial in maintaining quality, as they require SDOs to uphold principles of transparency, openness, balanced representation, and due process.
The Medicines and Healthcare products Regulatory Agency (MHRA) recently indicated an intent to acknowledge regulatory approvals from specific international jurisdictions, which emphasizes the significance of comprehending the global landscape of healthcare product regulations. This strategy aims to simplify the process, enabling the effective distribution of resources towards innovative products and facilitating faster patient access to secure and quality-assured healthcare tools.
Additionally, the Organization for Economic Cooperation and Development (OECD) has provided recommendations to assist companies in preventing their involvement in conflict by regulating their mineral sourcing methods, which is relevant to manufacturers working with substances like tin, tantalum, and tungsten that are utilized in healthcare tools. As the healthcare equipment market evolves, comprehending the particular governing setting where equipment are sold, imported, and used becomes imperative. Certain rules are confined to specific countries or regions, thus a worldwide compliance approach is frequently embraced to navigate the constantly evolving market strategies and governing landscapes.
Apart from following regulations, creativity and uniformity are promoted through the synchronization of guidelines, which is demonstrated by the flourishing technology sector in Michigan, acknowledged for its expertise and production capacities. The recent launch of equipment testing in UL Solutions' Rochester Hills laboratory is a testament to the industry's commitment to advancing the security, reliability, usability, and interoperability of healthcare devices.
This all-encompassing method of conformity assessment guarantees that healthcare instruments fulfill the utmost criteria of well-being and effectiveness, promoting confidence among healthcare providers, patients, and regulatory entities, and ultimately adding to the progress of healthcare technology.
Clinical Investigations and Evaluation
Clinical investigations play a vital role in the process of medical equipment development, guaranteeing that new technologies not only adhere to strict requirements but also prioritize the utmost patient well-being. These investigations encompass a range of activities, from the meticulous assessment of clinical data, whether sourced directly by the manufacturer or independently, to the ethical considerations that underscore the entire process. For example, the utilization of the Impella Connect System demonstrates the complex combination of hardware and software functions that necessitate premarket authorization, emphasizing the need for comprehensive clinical evaluation to confirm effectiveness and efficiency, particularly in critical care settings.
Clinical evaluations are more than a hurdle; they are a commitment to healthcare excellence. The creation of a Clinical Evaluation Report (CER) is a careful task that showcases the well-being of a healthcare apparatus in accordance with governing requirements. It entails a thorough exploration into the performance of the apparatus based on clinical evidence, a procedure that is not just regulatory in essence but also a moral obligation to safeguard patient safety. This continuous process of assessment and reassessment helps to navigate the constantly changing landscape of regulations for healthcare equipment, with transparency in the distribution of information about the rationale and comprehensibility of equipment functions being of utmost importance.
Moreover, the medical technology market's swift expansion, with the United States projected to generate a revenue of US$215.80bn by 2024, is indicative of the sector's pivotal role in healthcare. The market spans an impressive array of devices—from the simplicity of thermometers to the complexity of MRI machines and surgical robots, all of which undergo rigorous clinical investigations. These instruments are essential for patient care, and their advancement is motivated by an increasing need for advanced healthcare solutions and a worldwide emphasis on improving healthcare infrastructure. The variety of this market is equalled by the thoroughness of clinical investigations, which ensure the protection not only of the functional integrity of these instruments but also the health of the patients they serve.
Technical Documentation and Design Dossier
The development of technical documentation for healthcare instruments is a complex procedure that acts as the foundation for verifying adherence to regulatory guidelines. This documentation includes important elements like the design dossier, which thoroughly documents the development and capability of the product. Furthermore, it plays a crucial role in risk management and post-market surveillance, ensuring that both the quality and safety of the medical equipment are consistently monitored and maintained.
Highlighting the importance of user-focused design, it is crucial to take into account the diverse group of individuals who engage with the equipment, from patients to the multitude of healthcare professionals. The design process must cater to their unique needs and preferences to foster a positive emotional interaction, which can ultimately lead to better adoption, greater compliance, and enhanced clinical outcomes. This approach is backed by thorough user research and usability testing, guaranteeing that the product aligns with the desired user experience.
The medical industry is experiencing a rise in requirements, requiring a preparedness to adjust and effectively handle documentation to meet these changing standards. Transparency, as emphasized by the OECD's Conflict Minerals policy, requires a clear understanding of an object's intended use, development, and performance. This level of clarity ensures that risks and patient outcomes are effectively communicated to all stakeholders.
Consensus standards, developed through the collaboration of Standards Development Organizations, play a pivotal role in ensuring quality standards. These standards follow principles of transparency and inclusivity and are essential for a robust conformity assessment, which is the process of demonstrating that a product meets specified requirements. The incorporation of such agreement standards into the regulatory structure is crucial in protecting the honesty and adherence of healthcare instruments.
To summarize, the thorough creation of technical documentation and commitment to openness and agreement standards are essential to the lifecycle management of healthcare equipment. These practices not only promote adherence to regulations but also improve the overall quality and safety of the equipment, benefiting both users and patients alike.
Quality Management Systems and Good Manufacturing Practice (GMP)
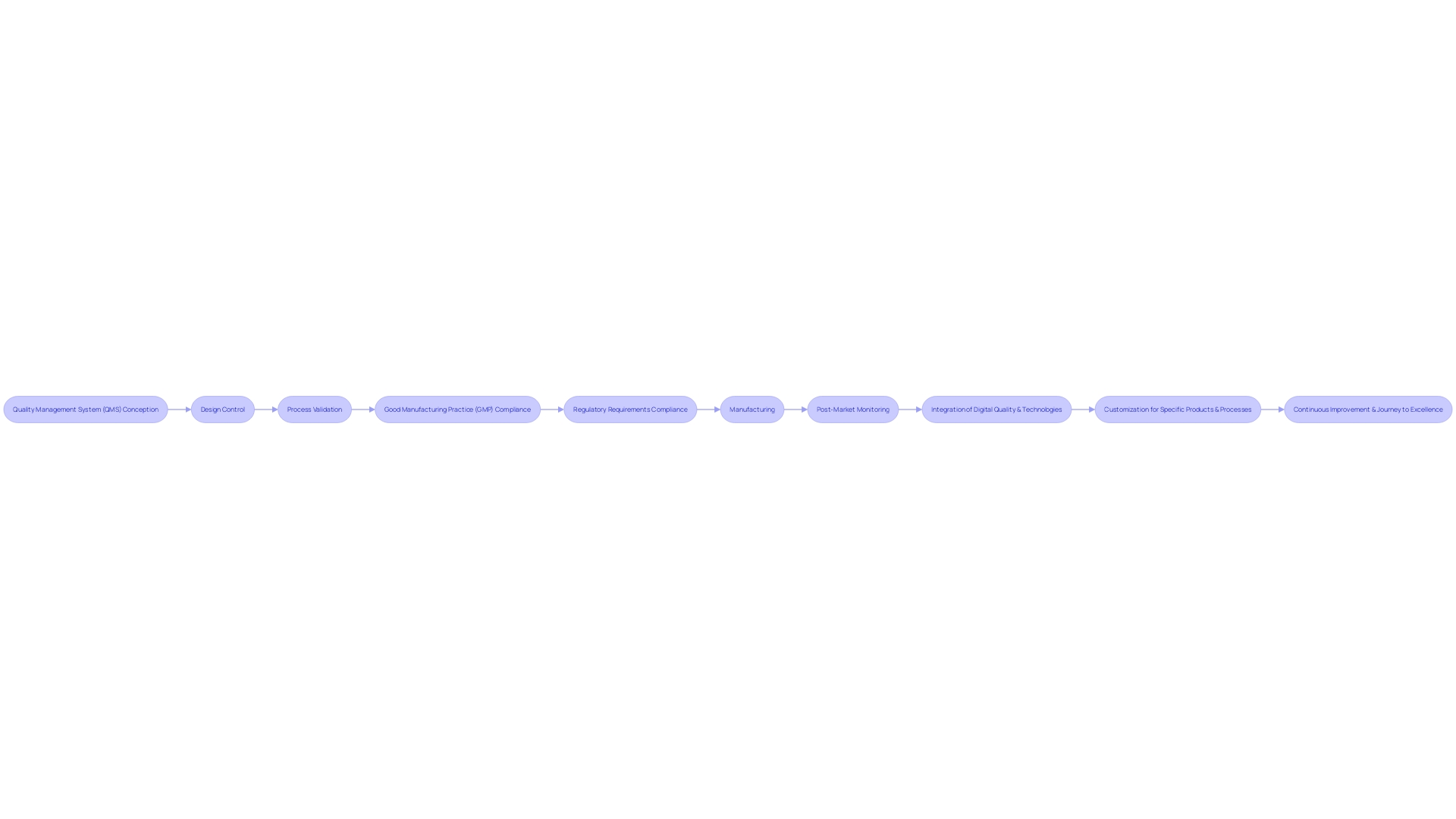
The healthcare equipment industry is characterized by an incredible diversity of products, ranging from simple items like spectacles to complex technologies such as MRI machines and pacemakers. The spectrum of tools reflects the multifaceted nature of healthcare and includes a variety of instruments, machines, implants, in vitro reagents, and software, all designed with individual and instrument factors diversity in mind. Considering the intricacy and the sheer quantity of accessible healthcare equipment, mentioned by the World Health Organization to be over 10,000 varieties, the creation of a Quality Management System (QMS) is crucial. A thorough Quality Management System guarantees the quality and safety of healthcare equipment by regulating the entire life cycle, from conception and creation to manufacturing and post-market monitoring.
Quality assurance in the healthcare equipment sector is a methodical process that ensures equipment meet predetermined quality standards and regulatory requirements, while delivering safe and effective outcomes. Important elements of quality assurance involve design control to guarantee the product meets its intended requirements and that risks are mitigated, and process validation to confirm manufacturing processes are consistent and comply with quality norms.
Complying with Good Manufacturing Practice (GMP) is crucial for the validation of healthcare instruments, offering a structure to uphold consistent quality and conformity with international standards like ISO 13485 and FDA regulations. However, the qualification process can be filled with challenges such as constraints imposed by regulations and confidentiality issues, which can slow down efficiency and lead to an overemphasis on compliance at the expense of value.
The integration of digital quality and technologies into existing systems necessitates a clear definition of objectives and the development of an implementation plan. This strategic approach is crucial for the smooth implementation of automation and AI technologies, which are expected to play a significant role in the future of the healthcare equipment industry. As the industry navigates the intricate legal framework, particularly in light of increased automation due to the COVID-19 pandemic, manufacturers must ensure they have the right tools and partnerships to comply with the law.
In summary, a QMS that meets the regulations and meets customer expectations is essential in the industry. Manufacturers are responsible for meeting these requirements and providing evidence of compliance. The adaptability of the QS regulation permits manufacturers to customize specific procedures to their products and processes, guaranteeing each apparatus is secure and efficient for patient use.
Labeling and Packaging Requirements
Tagging and packaging play crucial roles in the safety and effectiveness of healthcare tools, serving as gatekeepers of essential information for users and healthcare professionals. Labels are public facing and can be as crucial as the product itself, often being the first line of communication about the device's use, risks, and benefits. The Food and Drug Administration (FDA) emphasizes the importance of clear, conspicuous, and neutral presentation of information, including potential side effects, to ensure users make informed decisions.
Regulatory bodies, including the FDA and the European Union, require that labeling systems undergo rigorous process validation. This validation ensures that every step in the label design and printing process produces reliable outputs that comply with quality standards. Manufacturers must demonstrate that their systems are correctly installed, operate as intended, and perform safely, often through Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) activities.
The labeling requirements are supplemented by voluntary consensus standards developed by Standards Development Organizations (SDOs). These standards align with principles of transparency, balance, and due process, fostering innovation and standardization in healthcare technologies. They play a crucial role in the regulatory framework, ensuring that products adhere to high-quality norms and are consistently effective and safe for use. The consensus standards also extend to the evaluation of conformity assessment procedures, which include testing, inspection, and certification processes.
Furthermore, in order to support producers, particularly small and medium-sized businesses, the Commission and Member States have developed tables that outline the language prerequisites for instructions and labels of healthcare products in various nations. These resources are crucial for guaranteeing that equipment is accompanied by information that is accessible and comprehensible to users in their native languages, including the graphical user interface (GUI) for digital applications.
Considering the substantial amount of harm and fatalities that could be associated with healthcare tools, as stated in a 2018 examination of FDA information, the trustworthiness of labeling and packaging is not only a matter of rules but a crucial matter of public health. With continuous monitoring and enforcement measures against untrustworthy data submissions from third-party testing laboratories, regulatory agencies are dedicated to upholding trust and quality in the labeling and packaging of devices, thus guaranteeing that products adhere to the most stringent standards prior to reaching consumers.
Vigilance and Post-Market Surveillance
Ensuring the continuous well-being and efficiency of healthcare tools after their introduction into the market is a crucial aspect of healthcare. Establishing a robust vigilance system, along with comprehensive post-market surveillance (PMS), is imperative in maintaining high standards of patient care. PMS encompasses various monitoring strategies, including the reporting of adverse events and systematic trend analysis. These monitoring activities are intended to gather real-life data on equipment performance, thus enabling prompt interventions when concerns about well-being emerge.
In the context of healthcare application surveillance, 'High-Risk AI' systems, which include healthcare applications, require meticulous oversight due to their potential to cause widespread harm if misused. Taking cues from the exacting safety protocols of industries such as nuclear power and air travel, where evidence collection and stringent adherence to regulations are the norms, the regulation of medical devices must also adapt to ensure the well-being of the end users.
'Recent news highlights the UK's MHRA roadmap for regulation of healthcare equipment, with patient safety as a cornerstone.'. This new framework aims to enhance the UK's position as a leading environment for healthcare technology innovators while ensuring international harmonization of standards. The ongoing advancement of medical equipment technology, as observed by UL Solutions, emphasizes the necessity for continuous regulatory vigilance.
The FDA's definitions and regulations play a crucial part in the surveillance process, classifying equipment and monitoring alterations that may affect the well-being and efficiency. From the categorization of tools to the examination of promotional materials, each aspect is thoroughly assessed to safeguard consumer interests.
The importance of PMS is underscored by industry experts who stress the necessity of continuous evaluation beyond pre-market assessments. This includes utilizing both passive and active surveillance systems to gather data from healthcare professionals, patients, registries, studies, and electronic health records. These approaches contribute to a thorough comprehension of a healthcare instrument's long-term influence on patient well-being and instrument functionality.
In the end, the objective of watchfulness and monitoring after the product has been released is to guarantee that objects function as planned and do not present unexpected hazards to patients, with the dedication to adjust and react to new information on protection as it becomes accessible.
Unique Device Identification (UDI) and Traceability
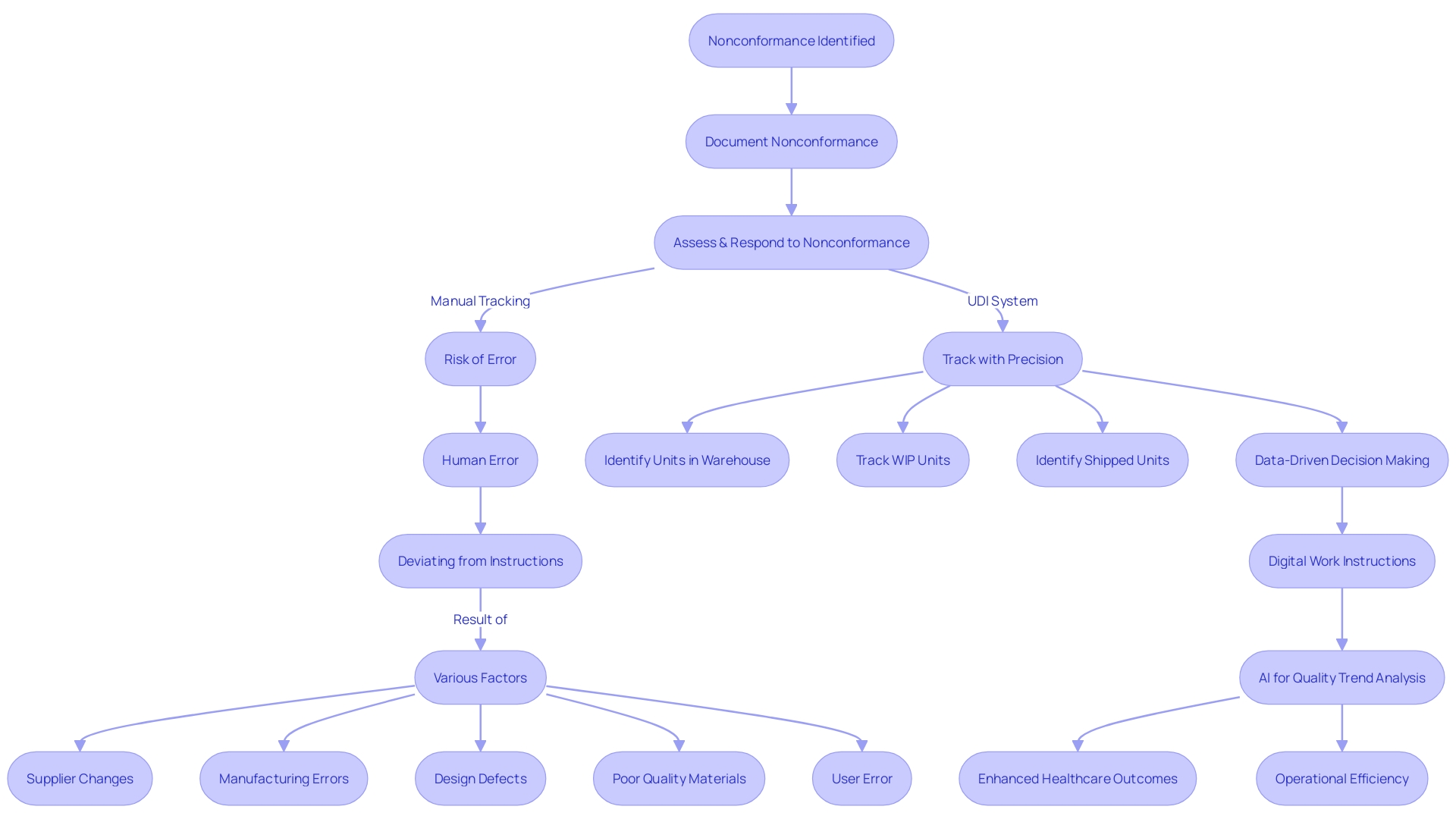
The adoption of a Unique Device Identification (UDI) system is a transformative step for medical device traceability and lifecycle management. The UDI system plays a pivotal role in mitigating risks associated with nonconformance, which can stem from various sources such as supplier changes, manufacturing errors, design flaws, substandard materials, or user errors. Nonconformances not only have negative effects on patient well-being, but they can also result in heightened healthcare expenses, strict penalties, and substantial product recalls.
By transitioning to a UDI system, manufacturers can move away from the perilous manual methods of managing nonconforming products at the serial number level, which are prone to errors in tracking items across warehouses, work-in-progress stages, and customer shipments. This shift is particularly crucial in addressing human errors and deviations from work instructions on the shop floor, which are among the most significant contributors to process nonconformance.
Moreover, the implementation of UDI facilitates a data-driven approach, enabling organizations to effortlessly access precise data for informed decision-making. It ensures digital continuity across the value chain, from engineering to supply chain and service functions, reducing time spent on information searches and providing reliable data and insights. The use of product data for generating digital work instructions and leveraging artificial intelligence to examine quality trends are further advantages of this approach.
As emphasized by industry experts with extensive experience in quality assurance and compliance affairs, such as Bruce McKean, Director of Regulatory at Rimsys, a UDI system is crucial for modern compliance and technical program strategies. It assists manufacturers in meeting the evolving global regulatory demands and enhances the overall quality management system, thereby safeguarding patient well-being and streamlining supply chain management. Ultimately, the UDI system is a valuable tool in the continuous effort to enhance healthcare outcomes and operational efficiency in the industry of medical equipment.
Custom-Made and Special Purpose Devices
Regulatory frameworks for custom-made and special purpose medical products are an essential part of maintaining the safety and effectiveness of these items. Under strict supervision, these equipment must meet documentation and labeling standards that assure conformity with legal requirements. Specific controls must be followed for items that are made to order or serve a distinct purpose. For example, such equipment must demonstrate compliance through rigorous conformity assessments, which may include testing, certification, and supplier declarations of conformity.
The FDA classifies equipment used in healthcare into three categories, with category three equipment being high-risk and subject to more rigorous processes due to their critical role in sustaining life, such as in implantable equipment like pacemakers. These tools, which are both customized and designed for specific purposes, need comprehensive documentation and precise labeling to effectively navigate the legal framework.
Voluntary consensus standards, developed or adopted by SDOs, play a pivotal role in the regulatory quality and the standardization of medical instruments. These standards are established by consensus and are essential for fostering innovation and facilitating patient access to new technology.
Furthermore, the industry is witnessing an alignment with Industry 4.0 principles, which emphasizes the integration of data sources and the use of supporting technologies to enhance production efficiency. This is especially applicable for custom-made and special purpose apparatus, which can benefit from these technological advancements for more personalized healthcare solutions.
The healthcare technology market is quickly expanding, with the United States projected to generate substantial revenue by 2024. This expansion highlights the significance of a framework that can adjust to the changing environment of healthcare tools, guaranteeing that custom-built and specific purpose tools persist in meeting the utmost standards of quality and security. By following these considerations, manufacturers can ensure their products are well-positioned to meet the market's demand while maintaining compliance with safety and effectiveness criteria.
Implementation Challenges and Best Practices
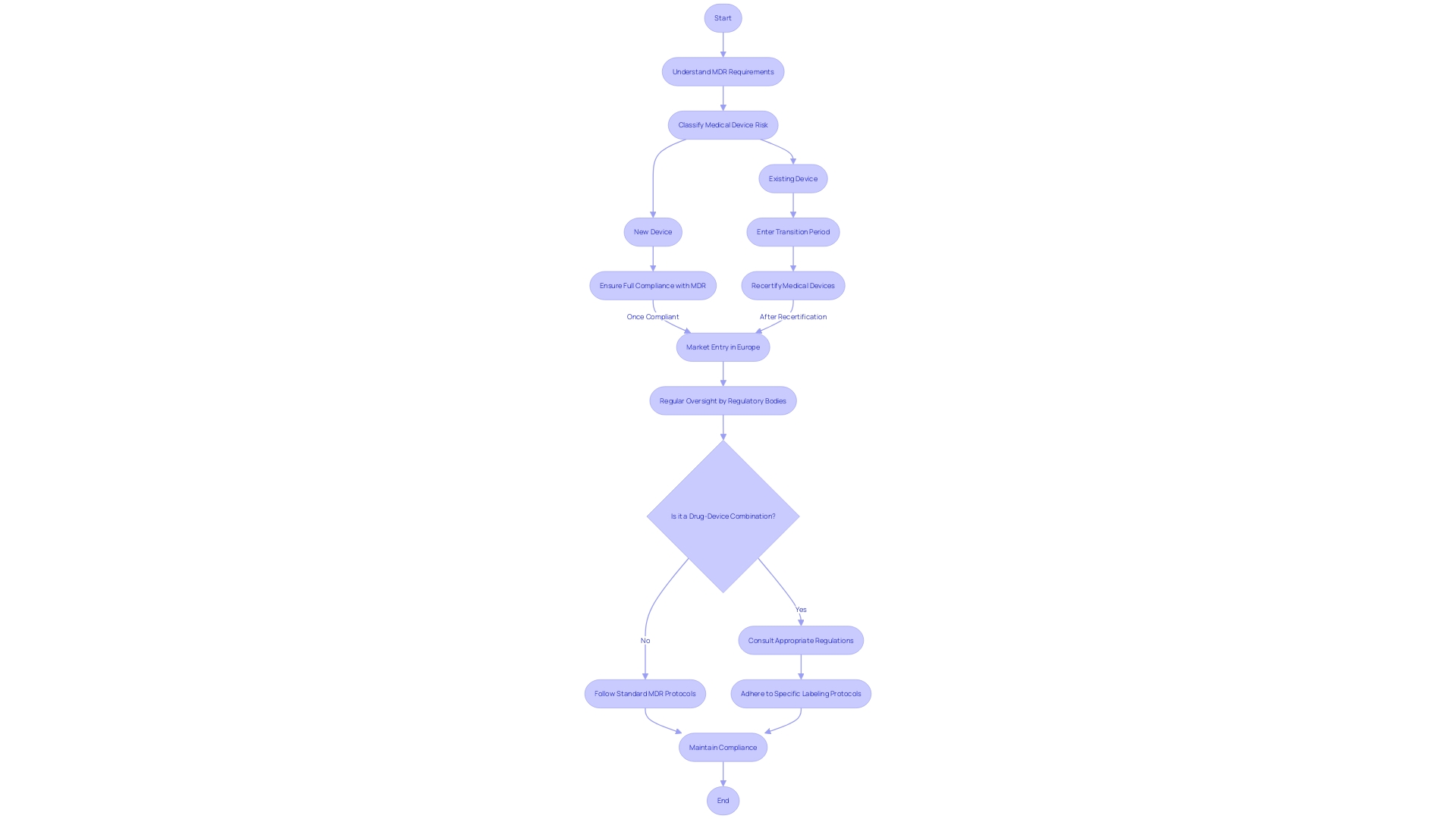
The field of regulating healthcare equipment is always changing, offering both difficulties and possibilities for makers and governing bodies alike. With the Medical Device Regulation (MDR) transforming the legal framework within Europe, compliance becomes a critical focus point. The MDR requires that all new healthcare instruments entering the European market adhere to updated requirements, while instruments already present must undergo recertification during a designated transition period.
Navigating the complexities of the MDR requires a strategic approach to ensure successful certification. This includes understanding the nuances of the regulations, such as those pertaining to integral drug-device combinations, like pre-filled syringes, and their lifecycle management. Furthermore, the instructions for co-packaged healthcare instruments, the labeling protocols, and the consultation procedures for instruments containing accompanying substances are all essential components of the compliance process.
As a result of the evolving regulations, healthcare technology is also progressing, leading to a wide range of tools that incorporate materials science, bioengineering, and information technology. As such, the demand for clear guidance on regulatory and procedural matters is at an all-time high. Recent updates aim to enhance openness within the EU and expedite the availability of healthcare equipment, especially those that are groundbreaking or cater to specific patient populations.
Effective compliance also involves a profound comprehension of the data generated by connected medical instruments and related services. Manufacturers must ensure that data, including crucial metadata, is accessible to users or designated third parties, while also providing fair and transparent access to other businesses when required.
The FDA and EMA continue to play crucial roles in the process, with the FDA categorizing products into classes based on potential risk and EMA involvement at the Member State level. The thoroughness of the processes for oversight varies, from simpler 510(k) clearances for low-risk items to more rigorous evaluations for high-risk class three items, which include life-sustaining implants like pacemakers.
For those in the industry, staying current with these regulations and practices is not merely a matter of legal compliance but a commitment to ensuring the safety and efficacy of medical devices across the globe. As the regulatory landscape evolves, so too must the strategies employed to navigate it, with a focus on continuous improvement and adaptability to new regulatory challenges.
Conclusion
In conclusion, the European Union's implementation of the Medical Device Regulation (MDR) and the In Vitro Diagnostic Regulation (IVDR) demonstrates a commitment to patient safety and innovation in the medical device sector. These regulations replace previous directives and establish updated requirements for market entry and compliance throughout a device's lifecycle. Manufacturers are adjusting their products to meet the stringent MDR requirements, prioritizing efficiency in regulatory documentation.
The EU is expediting the implementation of EUDAMED, a comprehensive database on medical devices, to enhance transparency. Industry reports highlight the impact of these regulations on device availability and strategies for maintaining compliance. Overall, the EU's implementation of the MDR and IVDR underscores its dedication to safeguarding public health while supporting innovation in the medical device sector.




