Introduction
Clinical trials are the cornerstone of assessing the safety and effectiveness of medical devices, providing crucial data for regulatory authorities and healthcare providers. These trials are not mere formalities; they are rigorous processes that validate device performance claims, uncover potential adverse effects, and ensure compliance with quality standards. The consequences of inadequate testing were evident in the case of Philips Respironics, where minimal human testing led to injuries and deaths.
This highlights the critical nature of comprehensive clinical trials in ensuring device safety. Moreover, clinical trials are part of a greater ecosystem that includes regulatory frameworks and post-market surveillance, emphasizing the need for robust trials to preemptively identify and mitigate risks. Clinical trials play a vital role in advancing medical treatments, as illustrated by Edwards Lifesciences' trial that tested a device to alleviate heart pressure and symptoms.
They also contribute to global health by offering potential lifesaving treatment options for patients with rare diseases, albeit with logistical and linguistic barriers. The narrative of clinical trials is one of continuous learning and adaptation, with thorough clinical validation remaining unchanged despite evolving standards. The world of medical device clinical trials is intricate and challenging, requiring diligent management and adaptability.
As clinical research evolves, the commitment to patient safety, regulatory compliance, and medical advancement remains steadfast.
Importance of Clinical Trials for Medical Devices
Clinical trials are the linchpin in assessing medical devices for safety and effectiveness, providing indispensable data to inform regulatory authorities and healthcare providers. These trials are not just a formality; they are a rigorous process of evidence collection that substantiates device performance claims, uncovers potential adverse effects, and verifies compliance with quality standards.
Take the case of Philips Respironics, for example, where a lack of thorough testing led to over 100,000 reports of injuries and numerous deaths. The recalled devices had undergone minimal human testing before market release, highlighting the critical nature of comprehensive clinical trials in ensuring device safety and efficacy.
Moreover, clinical trials are not isolated events but are part of a greater ecosystem that includes regulatory frameworks and post-market surveillance. The FDA's current constraints, as evidenced by the Respironics case, demonstrate the need for robust clinical trials that can preemptively identify and mitigate risks, rather than relying on post-market self-regulation by manufacturers.
To illustrate the importance of clinical trials in advancing medical treatments, consider Edwards Lifesciences' trial involving 100 patients across 30 sites, testing a device designed to alleviate heart pressure and symptoms like shortness of breath. The procedure involves a catheter inserted into a neck vein, showing the innovative approaches being tested to improve patient quality of life.
Clinical trials also play a vital role in global health. Patients with rare diseases sometimes face the need to travel internationally for potentially lifesaving treatment options, as in the hypothetical scenario of a patient from rural Pennsylvania needing to participate in a trial in Turkey. The logistical and linguistic barriers underscore the complexities and commitments required from patients and their families in the pursuit of advanced medical care.
The narrative of clinical trials is one of continuous learning and adaptation. Kaplan, a clinical research expert, notes that the FDA approval of a novel drug signals rigorous, monitored research studies. Despite the 21st Century Cures Act adjusting the standards for FDA approval, the essence of thorough clinical validation remains unchanged.
In closing, the world of medical device clinical trials is intricate and challenging, as Chris, a biomedical engineer with extensive experience in managing clinical studies, can attest. The dynamic nature of clinical trials—with shifts in personnel and procedural amendments—calls for diligent management and adaptability. As clinical research continues to evolve, the commitment to patient safety, regulatory compliance, and the pursuit of medical advancement remains steadfast.
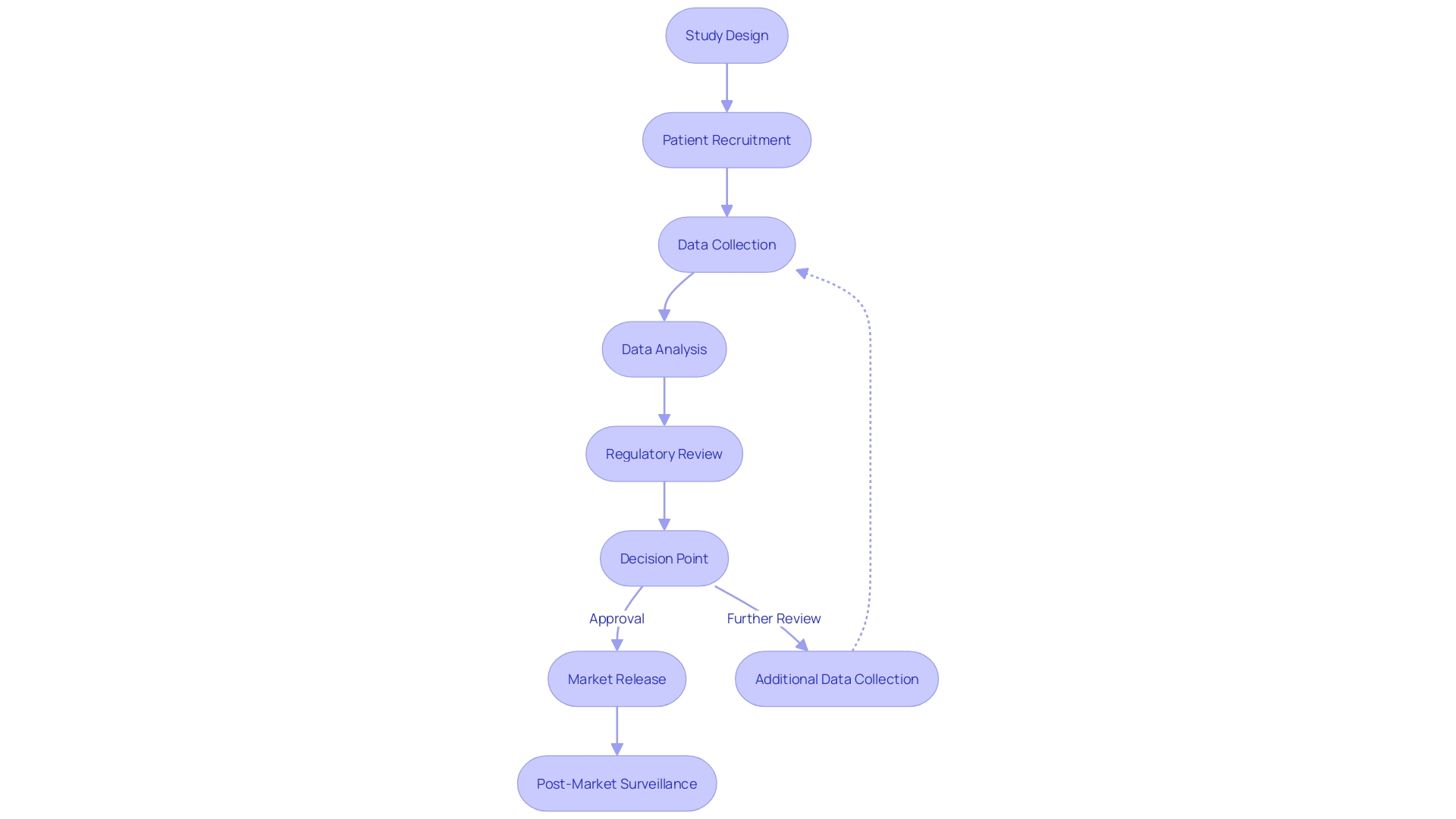
Preparation Phase
The meticulous groundwork involved in initiating a clinical trial is a multifaceted and critical process, encompassing several paramount activities that pave the way for a successful study. The design of the study is the blueprint that establishes the framework, including the identification of objectives, the selection of an appropriate study population, and the determination of endpoints. Alongside this, securing ethics approval from an ethics committee or institutional review board is a mandatory checkpoint that ensures the trial adheres to ethical standards.
Selecting the right investigators is equally crucial; these professionals are the linchpins who will steer the trial towards its goals, necessitating a careful match between the trial’s needs and the investigators' expertise. Similarly, site selection is a strategic decision that hinges on factors such as site capabilities, patient demographics, and infrastructure support for the trial.
The development of a trial protocol is perhaps the most intricate of these pre-trial tasks. It serves as a comprehensive manual, detailing the study design, the methodologies to be employed, and the procedures for data collection. This document is not just a guide but a cornerstone that upholds the integrity and efficacy of the clinical trial.
This rigorous preparation phase, informed by a practical understanding of safety concerns and tailored to address specific populations, is integral to the conscientious development of medical products. As highlighted by recent guidance on digital health technologies for remote data acquisition, the landscape of clinical trials is evolving, and staying abreast of these advancements is essential for all stakeholders involved, including healthcare professionals and researchers.
Indeed, clinical trials are a testament to the progress in medical research, aimed at testing and establishing the safety and effectiveness of new treatments or interventions. As they progress through their phases—from the safety-focused phase one trials to the more expansive phase two trials evaluating both efficacy and safety—these studies are instrumental in shaping patient care and outcomes. With each trial meticulously crafted and executed, the collective goal remains steadfast: to advance medical knowledge and improve the lives of patients worldwide.
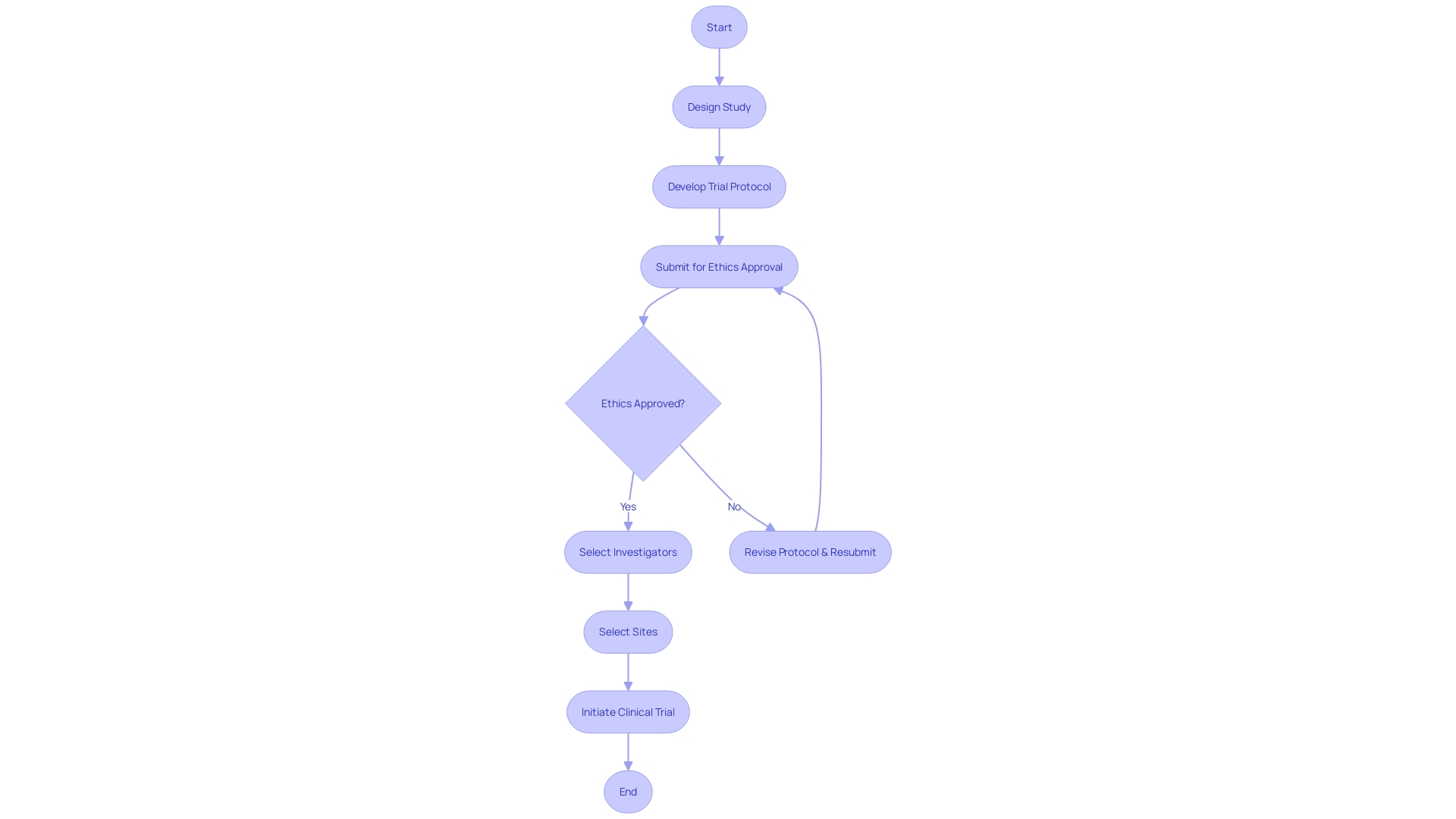
Regulatory Requirements and Guidelines
Upholding rigorous standards during the execution of clinical trials is not only a regulatory mandate but also a moral imperative to ensure participant safety and data integrity. As the landscape of medical research evolves, with trials spanning global boundaries and incorporating advanced technologies such as AI and ML, the scrutiny on compliance has intensified. Regulatory authorities like the FDA and EMA diligently oversee the enforcement of strict protocols that encompass informed consent, safety reporting, and data accuracy.
Their vigilance is mirrored by industry leaders such as AstraZeneca, who navigate these guidelines while advancing therapeutic innovations.
The MHRA, exemplifying regulatory excellence, aids sponsors with guidance and support, aiming to streamline the application process for clinical trial authorizations. This proactive engagement helps circumvent common pitfalls in compliance and is indicative of a broader regulatory trend towards facilitation rather than mere enforcement.
In the U.S., the FDA's commitment to public health is further exemplified by its recent rule on transparent communication in direct-to-consumer drug advertisements. This ensures that the benefits and risks of new treatments are conveyed clearly, fostering informed decision-making among patients.
The significance of regulatory adherence extends beyond legal obligations; it is the foundation upon which the trust between the public, healthcare providers, and research institutions is built. In ensuring that new interventions are safe and efficacious, clinical trials serve as the critical pathway to improving patient care and outcomes, particularly in the challenging context of rare diseases and conditions with limited treatment options. The collective effort of regulatory bodies, sponsors, and healthcare professionals in maintaining these standards underpins the integrity of clinical trials and ultimately contributes to the advancement of medical science and the betterment of patient lives.
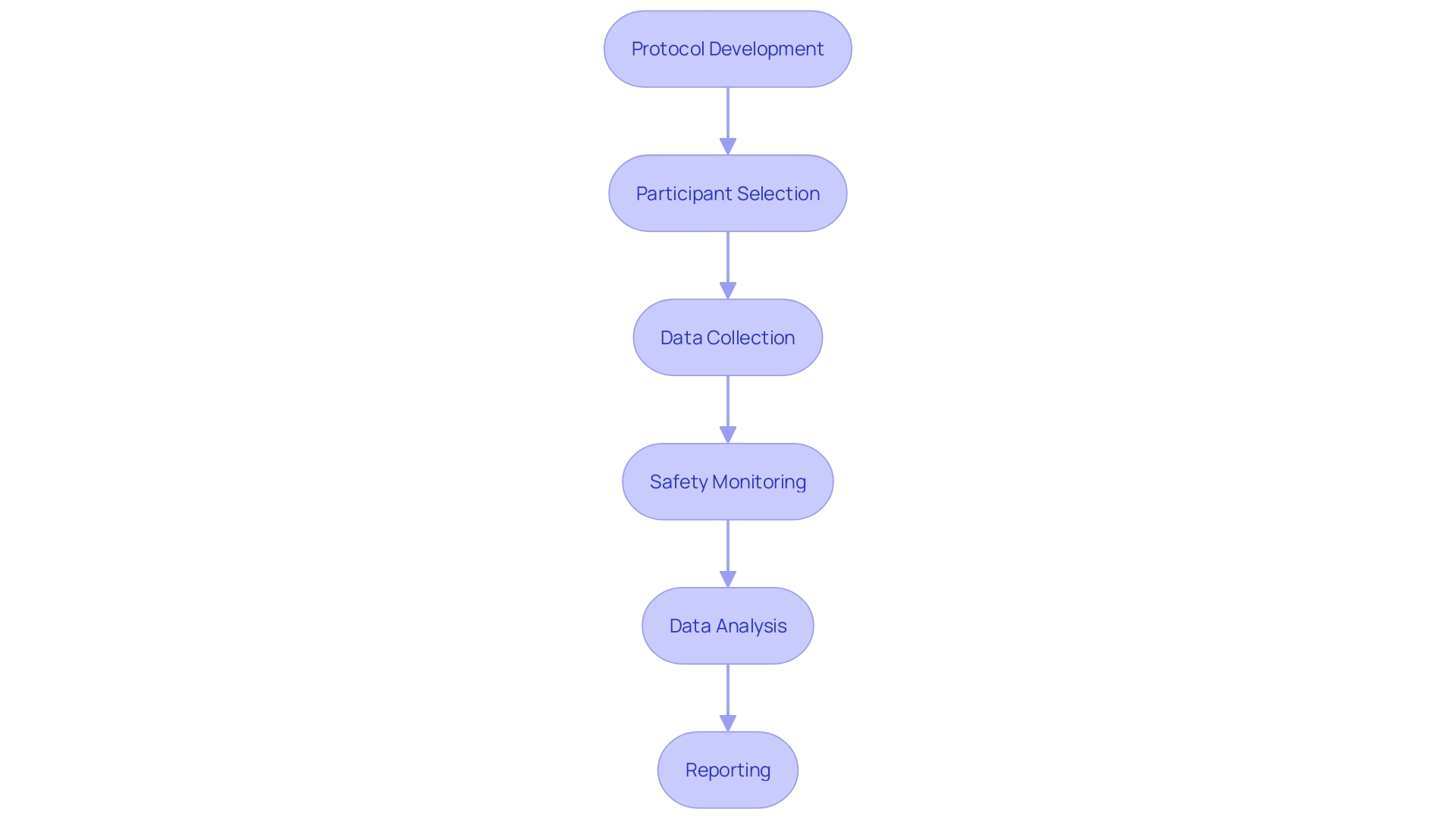
Step-by-Step Checklist for Conducting Clinical Trials
- Protocol Development:
- Establishing clear trial objectives, selecting a study population, and defining specific endpoints are paramount.
-
Crafting the study methodology and calculating the necessary sample size are critical for the trial's integrity.
-
Participant Selection and Informed Consent:
- Creating precise eligibility criteria to select appropriate participants is essential for the study's relevance.
-
It is important to ensure that informed consent is ethically obtained from all participants, honoring their autonomy and understanding of the trial.
-
Data Collection and Management:
- Choosing suitable data collection methods and tools is fundamental to gather accurate and reliable data.
-
A robust data management system is vital to handle the complexities of clinical trial data efficiently.
-
Safety Monitoring and Adverse Event Reporting:
- A comprehensive safety monitoring plan is essential to protect participants throughout the trial.
-
Adverse events and safety concerns must be reported promptly to maintain the trial's integrity and participant safety.
-
Data Analysis and Interpretation:
- Data must be analyzed with appropriate statistical methods to ensure valid results.
-
The interpretation of results should lead to meaningful conclusions that contribute to the field of medical research.
-
Reporting and Submission:
- A detailed clinical study report is crucial to document the trial findings comprehensively.
- Regulatory authorities require the submission of this report to ensure compliance and to potentially shape future healthcare policies.
By methodically following these steps, clinical trials can be conducted with the utmost precision, thereby contributing valuable insights into medical research and patient care.
Common Mistakes to Avoid
When planning a clinical trial, comprehensive preparation is paramount to avoid unnecessary delays, resource wastage, and compromised data integrity. It's essential to meticulously outline each stage of the trial to ensure a streamlined process. Participant recruitment poses another significant challenge, as insufficient enrollment can undermine the trial's statistical power and its broader applicability.
Strategies to attract and retain eligible participants are vital for robust results.
Data management is another critical aspect; without rigorous data collection and management protocols, the validity of trial outcomes is at risk. It's crucial to have a robust system in place that safeguards data integrity. Moreover, communication and collaboration among stakeholders cannot be overstated; they are the linchpins of a successful clinical trial.
It's important to establish unambiguous communication channels and foster a collaborative environment to facilitate smooth operations.
A recent study, published in the journal Facets, highlighted the lack of public records for many clinical trials, discussing the potential consequences of such oversight. With only 59% of trials registered before participant enrollment, and 32% of studies not reporting results or publishing findings, the study underscores the importance of transparency and thorough reporting in clinical research.
Furthermore, the complexities of modern clinical trials, such as patients managing logistics for international studies or the use of up to 20 different systems by clinical research sites, emphasize the need for streamlining operations. Better coordination and support can mitigate these challenges, as demonstrated by a project that effectively integrated electronic health record data into a multi-center trial.
In conclusion, by recognizing and proactively addressing these common pitfalls, one can significantly enhance the likelihood of conducting a successful clinical trial that adheres to the highest standards of research quality and ethical practice.
Best Practices for Successful Clinical Trials
Clinical trial companies are at the forefront of medical innovation, but their success hinges on several core practices:
-
Collaboration: A collaborative approach is fundamental. For instance, EHR-sourced trials have shown that leveraging electronic health records can optimize existing trial sites and infrastructures to meet study objectives. This requires the cooperation of various stakeholders, including investigators, sponsors, regulatory bodies, and ethics committees, to ensure that trials are not only compliant with regulations but also beneficial for patient outcomes. Such partnerships, as exemplified by Gabriel Jones' work, have already made significant strides in areas like improving pregnancy outcomes globally.
-
Effective Communication: Clear communication is imperative, as it ensures that all parties involved, from researchers to participants, are informed and engaged. This aspect became particularly evident in scenarios involving patients from remote areas who are required to travel internationally for clinical trials. Addressing their logistical concerns and ensuring they understand the process is crucial for their involvement and the trial's success.
-
Continuous Training and Education: Staying abreast of the latest trends, regulations, and best practices is essential for clinical trial companies. By continuously educating their teams, these companies can better navigate the complexities of clinical research and adapt to the evolving landscape, thereby enhancing the quality and relevance of their trials.
-
Quality Assurance: Implementing robust quality assurance processes is critical to maintaining data integrity and trial quality. The importance of this is reflected in the extensive scrutiny that trial results receive upon publication in journals like The Joint Commission Journal on Quality and Patient Safety, which emphasizes the need for high-quality improvement interventions and methodologies.
The stakes are high in clinical research, as evidenced by the statistics showing that 80% of clinical trials do not finish on schedule, often due to patient recruitment and retention challenges. By adhering to these best practices, clinical trial companies can enhance their operational efficiency, leading to more reliable results and ultimately, improved patient outcomes.
Additional Resources and References
As professionals in clinical research, staying abreast of guidelines and regulations is crucial for the successful development and implementation of medical device clinical trials. A wealth of resources is at hand for those seeking comprehensive guidance:
-
The FDA's guidance documents offer a deep dive into regulatory requirements ensuring the safety, effectiveness, and security of medical devices. These documents are essential for understanding the nuances of medical device approval and post-market surveillance.
-
The International Council for Harmonisation (ICH) Guidelines provide a framework for harmonizing the technical requirements for pharmaceuticals and medical devices, facilitating a more streamlined process internationally.
-
ClinicalTrials.gov serves as a repository for information on publicly and privately supported clinical studies on a wide range of diseases and conditions, including those involving medical devices.
-
ISO 14155:2020 is the gold standard for good clinical practice in the design and execution of clinical trials involving medical devices, ensuring the rights, safety, and well-being of trial subjects are protected.
Understanding the multifaceted nature of medical device clinical trials is further exemplified by the pathway to market for machine learning/artificial intelligence medical devices (Names). Similar to pharmaceuticals, Names undergo rigorous lab development, safety, and efficacy testing, and must achieve commercial success in a competitive market. The process is complex and requires not only technological innovation but also a strategic approach to clinical evidence and regulatory compliance.
For instance, the FDA's recent town hall discussions on medical device sterilization and exemptions to reporting requirements highlight the evolving landscape of medical device regulations and the importance of real-world data. Additionally, the FDA's Breakthrough Devices Program and new guidance on computer software assurance are indicative of the agency's commitment to promoting health equity and the efficient regulation of innovative medical technologies.
These resources, combined with an understanding of current trends and regulatory updates, can empower researchers to navigate the intricacies of medical device clinical trials. They enable the formulation of robust hypotheses, the use of historical controls, the re-training of AI/ML-enabled devices, and the generation of clinical evidence necessary for marketing authorization. Furthermore, they underscore the responsibility to protect confidential information and the importance of public commentary in shaping regulatory practices.
Amidst this dynamic regulatory environment, it's essential to consider the perspectives of industry experts like Etienne Nichols, who bring valuable insights into the integration of systems within medical devices. Their experiences bridge the gap between manufacturing, product development, and regulatory compliance, shedding light on the practical aspects of bringing medical devices to the market.
By leveraging these resources and insights, clinical research professionals can ensure that their work not only meets but exceeds the high standards set for medical device trials, ultimately advancing patient care and medical innovation.
Conclusion
Clinical trials are crucial for assessing the safety and effectiveness of medical devices. They validate device performance claims, uncover potential adverse effects, and ensure compliance with quality standards. The case of Philips Respironics highlights the critical nature of comprehensive clinical trials in ensuring device safety.
Clinical trials are part of a greater ecosystem that includes regulatory frameworks and post-market surveillance, emphasizing the need for robust trials to identify and mitigate risks.
These trials also play a vital role in advancing medical treatments. They offer potential lifesaving treatment options for patients with rare diseases, despite logistical and linguistic barriers. The narrative of clinical trials is one of continuous learning and adaptation.
Thorough clinical validation remains unchanged, even as standards evolve.
The world of medical device clinical trials is intricate and challenging, requiring diligent management and adaptability. As clinical research evolves, the commitment to patient safety, regulatory compliance, and medical advancement remains steadfast. In conclusion, clinical trials are essential for assessing medical devices, ensuring safety, advancing treatments, and contributing to global health.
The commitment to patient safety and medical advancement remains unwavering.




