Introduction
Navigating the regulatory landscape of medical devices is a complex and critical process, particularly when it comes to ensuring the safety and effectiveness of high-risk Class III devices. The Premarket Approval (PMA) process, overseen by the U.S. Food and Drug Administration (FDA), represents the most stringent pathway for device approval, necessitating comprehensive evidence from manufacturers. This article delves into the intricacies of the PMA process, contrasting it with the 510(k) pathway, and explores the FDA's classification system for medical devices.
Furthermore, it outlines the key components of a PMA application, details the four-step review process, and examines the potential actions the FDA can take upon reviewing a PMA application. Understanding these regulatory requirements is essential for businesses aiming to bring innovative and safe medical devices to market while adhering to the highest standards of public health protection.
What is PMA?
Premarket Approval (PMA) is a stringent regulatory procedure through which the U.S. Food and Drug Administration (FDA) assesses the safety and effectiveness of Class III medical instruments. Unlike the 510(k) process, which permits the marketing of products based on substantial equivalence to an already approved product, PMA demands comprehensive evidence from manufacturers. This evidence must demonstrate that the apparatus is safe for its intended use and effective in achieving its intended results.
The necessity for such stringent regulations is emphasized by alarming statistics: over a recent ten-year period, faulty medical instruments have been potentially linked to more than 1.7 million injuries and 83,000 deaths in the U.S. These tools range from everyday items like surgical masks to life-sustaining implantable pacemakers. 'In response, the FDA has been actively developing a surveillance system to monitor these products' well-being, starting with a few and expanding over time, despite facing challenges in funding and patient identification.'.
As part of the PMA process, manufacturers often need to conduct post-approval studies to ensure ongoing security and effectiveness. These studies may investigate long-term performance data that wasn’t available at the time of initial approval. Reports from these studies, which must be submitted at specified intervals, include overviews, progress summaries, and interpretations of the data collected. Furthermore, any changes to the equipment that could affect its safety and effectiveness require a PMA supplement, which must be approved by the FDA before implementation.
For businesses navigating these regulatory pathways for the first time, understanding the distinctions between the 510(k) and PMA processes can be challenging. Each pathway is based on the item’s risk class, which is determined through the FDA’s classification database. For instance, a catheter balloon repair kit is classified as a Class III cardiovascular instrument. By identifying the proper classification, businesses can better understand the level of FDA review required and ensure compliance with regulatory standards.
In summary, the PMA process signifies the FDA’s dedication to safeguarding public health by ensuring that high-risk medical products undergo thorough evaluation before reaching the market.
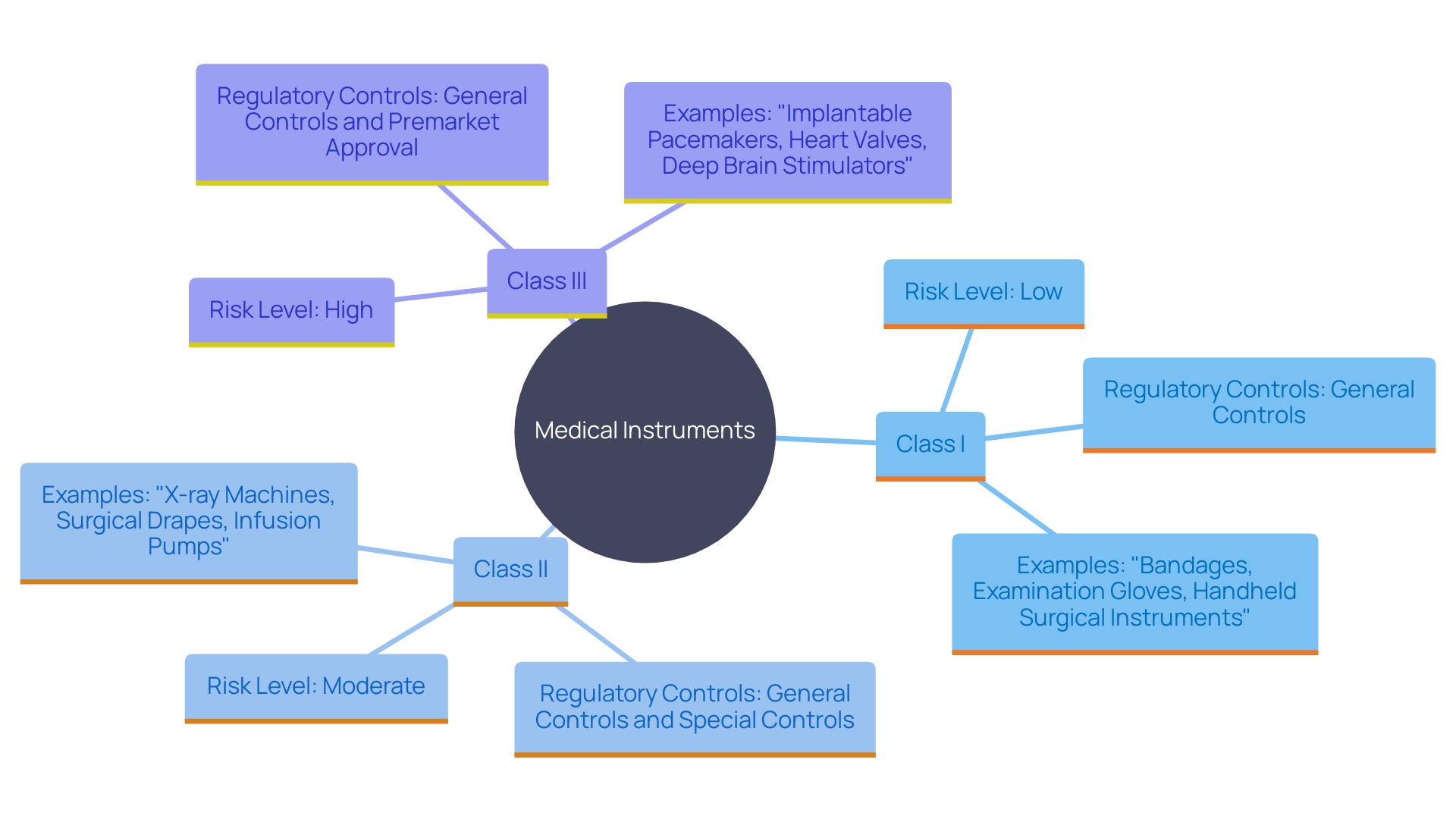
FDA Medical Devices Classification
The FDA categorizes medical instruments into three groups according to the level of risk linked to their usage: Class I, Class II, and Class III. Class I items pose the lowest risk and are subject to minimal regulatory control, primarily ensuring that these products adhere to general controls such as proper labeling and manufacturing practices. Class II items, which involve a moderate level of risk, require greater regulatory oversight. These instruments typically undergo a 510(k) premarket notification process to demonstrate substantial equivalence to a legally marketed product. Class III items represent the highest risk, often including life-sustaining or implantable products. These instruments must undergo the stringent Premarket Approval (PMA) process to provide valid scientific proof of their reliability and effectiveness before they can be sold.
The FDA's rigorous categorization and authorization procedures are essential in upholding high standards for equipment reliability and performance. For example, over 1.7 million injuries and 83,000 fatalities during a recent decade in the U.S. have been possibly associated with defective medical equipment. This highlights the importance of thorough scrutiny and regulation. The FDA has even started developing a monitoring system to track possible concerns regarding medical instruments, beginning with a few and growing gradually, to ensure continuous product security and adherence.
Key Components of the FDA PMA Application
A PMA application is an exhaustive document that comprises several critical components essential for the FDA's thorough evaluation. This encompasses clinical data showing the safety and effectiveness of the product, detailed descriptions of the item and its intended application, manufacturing information, labeling, and summaries of both clinical and nonclinical studies. 'This comprehensive documentation is vital, especially considering that more than 1.7 million injuries and 83,000 deaths over a recent 10-year period in the U.S. have been potentially linked to faulty medical equipment.'. The FDA’s surveillance system and stringent evaluation process aim to mitigate such risks, ensuring that only high-quality products reach the market. Furthermore, it is essential for producers to follow applicable guidelines, such as ISO 14971, to improve patient protection and aid in the progress of the medical equipment sector. As Dr. John Sheets, former head of the FDA’s Office of Device Evaluation, emphasized, pre-submission meetings with the FDA are pivotal steps towards PMA acceptance, providing opportunities for feedback and ensuring that submissions meet all regulatory requirements.
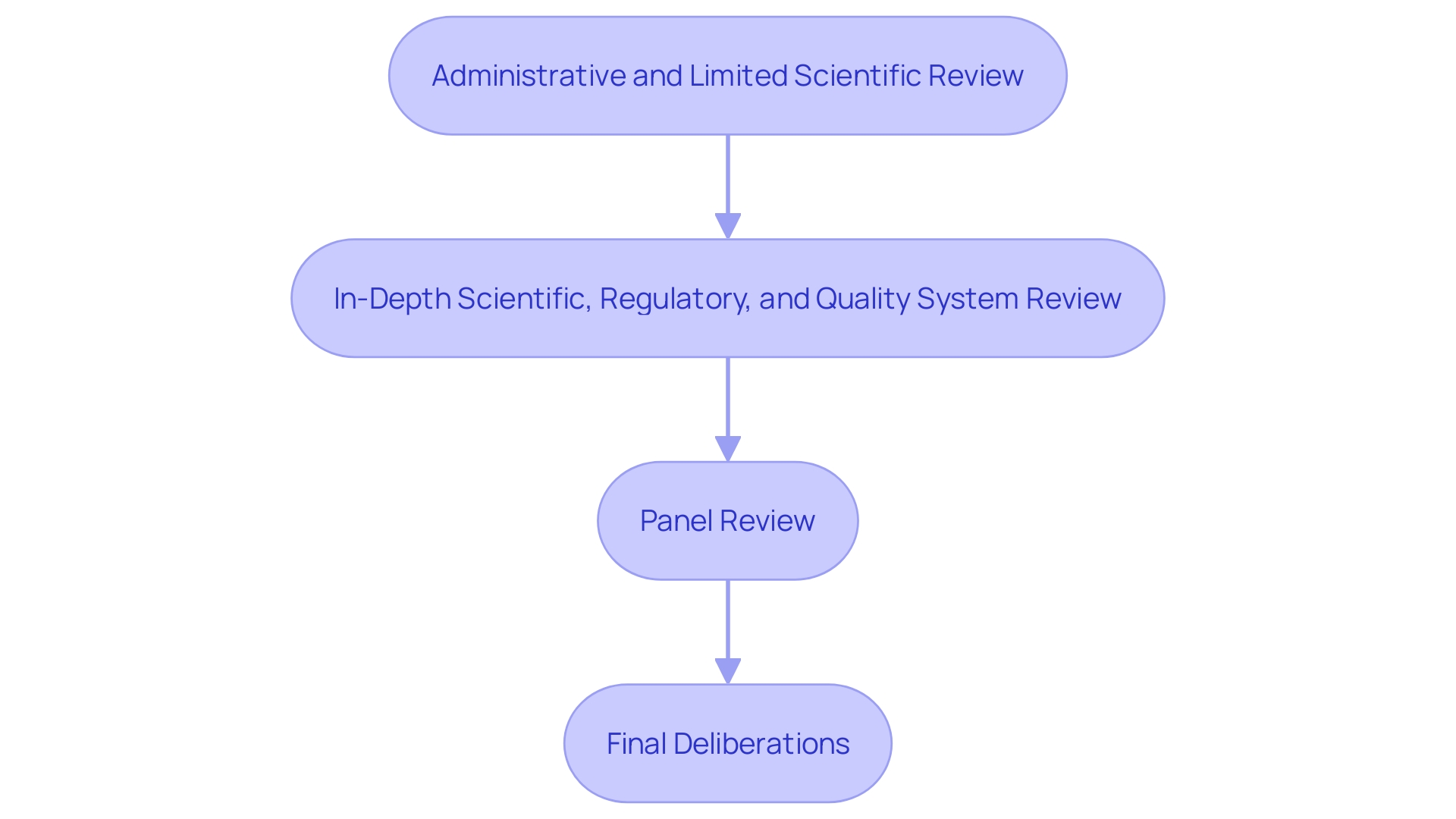
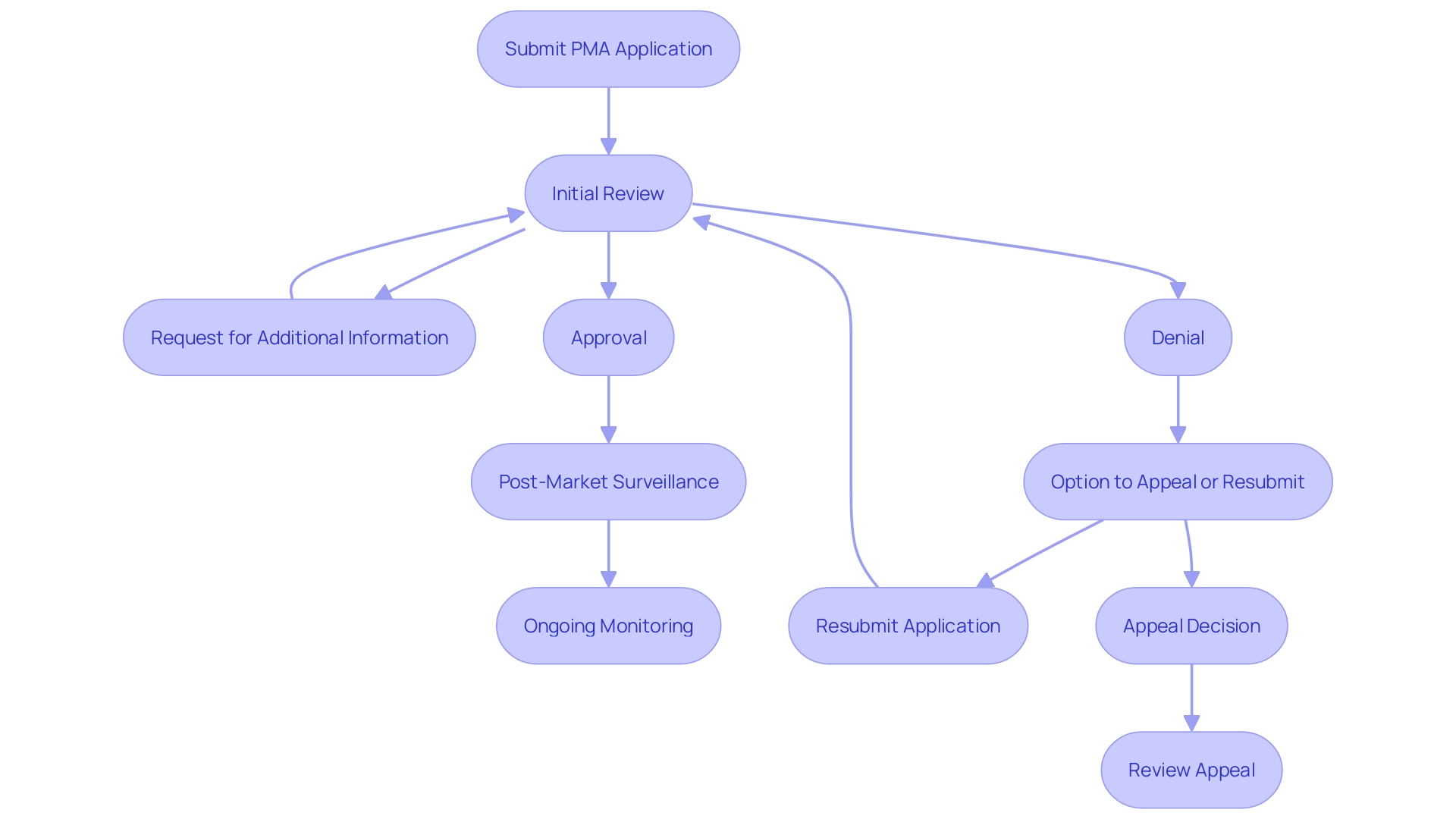
The 4-Step PMA Review Process
The PMA review process comprises four critical stages:
-
Administrative and Limited Scientific Review: This initial step involves verifying whether the application is complete and ready for a more detailed review. The FDA notifies the applicant within 45 days if the application is fileable; if not, reasons for non-acceptance and steps for resubmission are provided.
-
In-Depth Scientific, Regulatory, and Quality System Review: At this stage, a comprehensive evaluation of the product's safety and effectiveness is conducted. This review includes an examination of the apparatus's indications for use, its functional components, and adherence to performance standards. The methods, facilities, and controls used for manufacturing, processing, packaging, and storing the item are also scrutinized to ensure compliance with regulatory standards.
-
Panel Review: An advisory committee reviews the application and provides recommendations based on their expertise. This panel review is crucial for evaluating the long-term performance data that may not have been available at the time of initial approval.
-
Final Deliberations, Documentation, and Notification of FDA Decision: The final phase involves comprehensive documentation and a decision by the FDA. 'The decision-making process is supported by post-market surveillance data, which is essential for monitoring the product's performance in real-world settings.'. The FDA's dedication to quality is clear in its continual efforts to align regulatory frameworks with global benchmarks, ensuring prompt introduction of secure and effective medical devices.
After approval, manufacturers are frequently obligated to carry out studies to ensure ongoing security and effectiveness. These post-approval studies must be meticulously reported, covering study progress, data regarding well-being, and an interpretation of results. Any modifications to the apparatus that affect its safety and effectiveness must be submitted as a PMA supplement prior to implementation.
The significance of these actions cannot be emphasized enough, particularly considering data indicating that more than 1.7 million injuries and 83,000 fatalities in the U.S. during a recent decade have been associated with defective medical equipment. The FDA's establishment of a surveillance system to monitor these issues underscores the critical nature of each review stage in the PMA process.
Possible FDA Actions on a PMA Application
When the FDA reviews a PMA application, it can take several actions, including approval, denial, or requesting additional information or studies. An approved product can be marketed; however, the FDA may impose post-market surveillance requirements to monitor its performance in real-world settings. This is essential as over 1.7 million injuries and 83,000 fatalities during a recent 10-year span in the U.S. have been potentially connected to defective medical equipment, ranging from surgical masks to implantable pacemakers. The FDA is actively constructing a monitoring system to identify potential risk issues, though it faces challenges such as funding and patient identification. If a PMA application is denied, the manufacturer has the option to appeal the decision or modify the application based on FDA feedback. As Mike Drues from the Global Medical Device Podcast stated, “Just because you're meeting the standard, that just means that you're passing... That doesn't necessarily mean that you're making a safe and effective product.” This highlights the importance of rigorous and ongoing monitoring to ensure device safety and efficacy.
Conclusion
The Premarket Approval (PMA) process is a critical regulatory pathway for Class III medical devices, ensuring that only those demonstrating rigorous safety and effectiveness reach the market. By requiring comprehensive evidence from manufacturers, the FDA aims to mitigate the risks associated with high-risk devices, as evidenced by the alarming statistics linking faulty medical devices to significant injuries and fatalities. The necessity of stringent oversight is further emphasized by the FDA's ongoing efforts to establish a surveillance system to monitor device performance post-approval.
Understanding the distinctions between the PMA and 510(k) processes is essential for manufacturers. Each pathway is determined by the device's risk classification, which guides the level of regulatory scrutiny required. A thorough PMA application, encompassing clinical data, manufacturing practices, and adherence to international standards, is crucial for successful approval.
The four-step review process—comprising administrative checks, in-depth evaluations, panel reviews, and final deliberations—ensures that all aspects of device safety and effectiveness are thoroughly examined.
Upon reviewing a PMA application, the FDA can approve, deny, or request additional information, thereby underscoring the importance of ongoing compliance and post-market surveillance. The commitment to maintaining high standards of public health protection is evident in the FDA's rigorous evaluation process, which aims to ensure that high-risk medical devices are safe, effective, and continuously monitored for any potential issues. This comprehensive approach not only safeguards patients but also fosters innovation within the medical device industry.




