Introduction
Accelerated aging plays a pivotal role in the medical device industry by enabling manufacturers to estimate the shelf life and reliability of products under stress conditions. Through controlled simulations of time, temperature, humidity, and other environmental factors, manufacturers can project how devices will perform over extended periods. This process ensures that medical devices remain safe and effective, ultimately leading to improved health outcomes.
To keep up with stringent regulatory requirements, companies must adopt robust testing methodologies. The evolving regulatory landscape, shaped by initiatives like the OECD's Conflict Minerals policy, demands meticulous compliance efforts. The integration of digital technologies, such as AI and machine learning, further aids in enhancing the precision and efficiency of accelerated aging tests.
This technological advancement not only meets modern demands but also boosts productivity, ensuring that devices meet the highest safety standards.
In light of the increasing use of AI in medical devices, regulatory scrutiny has intensified. The recent guidelines released by the Biden administration and ongoing discussions in the European Parliament emphasize the need for compliance with AI-related regulations. This shift in regulatory focus necessitates that manufacturers remain vigilant and adaptable to maintain compliance and market competitiveness.
Why Accelerated Aging is Crucial for Medical Devices
'Accelerated aging plays a crucial role in the healthcare equipment sector by enabling producers to estimate the shelf life and reliability of products under stress conditions.'. Through controlled simulations of time, temperature, humidity, and other environmental factors, manufacturers can forecast how products will perform over extended periods. This process guarantees that medical instruments stay secure and efficient, ultimately resulting in better health results.
To keep up with the stringent regulatory requirements, companies must adopt robust testing methodologies. As Kevin Becker aptly stated, "All models are wrong; some models are useful." This pragmatic approach highlights the significance of employing effective models to forecast longevity and reliability accurately. Based on Bijan Elahi's extensive experience, comprehending and handling risks in medical equipment are essential, as outlined in his work on safety risk management.
The evolving regulatory landscape, shaped by initiatives like the OECD's Conflict Minerals policy, demands meticulous compliance efforts. As emphasized by industry specialists, this involves remaining informed about regulations that pertain to the local and international markets in which the products are marketed. The integration of digital technologies, such as AI and machine learning, further aids in enhancing the precision and efficiency of accelerated aging tests. 'This technological advancement not only meets modern demands but also boosts productivity, ensuring that equipment meets the highest safety standards.'.
'In light of the increasing use of AI in medical devices, oversight scrutiny has intensified.'. 'The recent guidelines released by the Biden administration and ongoing discussions in the European Parliament emphasize the need to adhere to AI-related regulations.'. This shift in regulatory focus necessitates that manufacturers remain vigilant and adaptable to maintain compliance and market competitiveness.
Understanding Accelerated Aging Tests
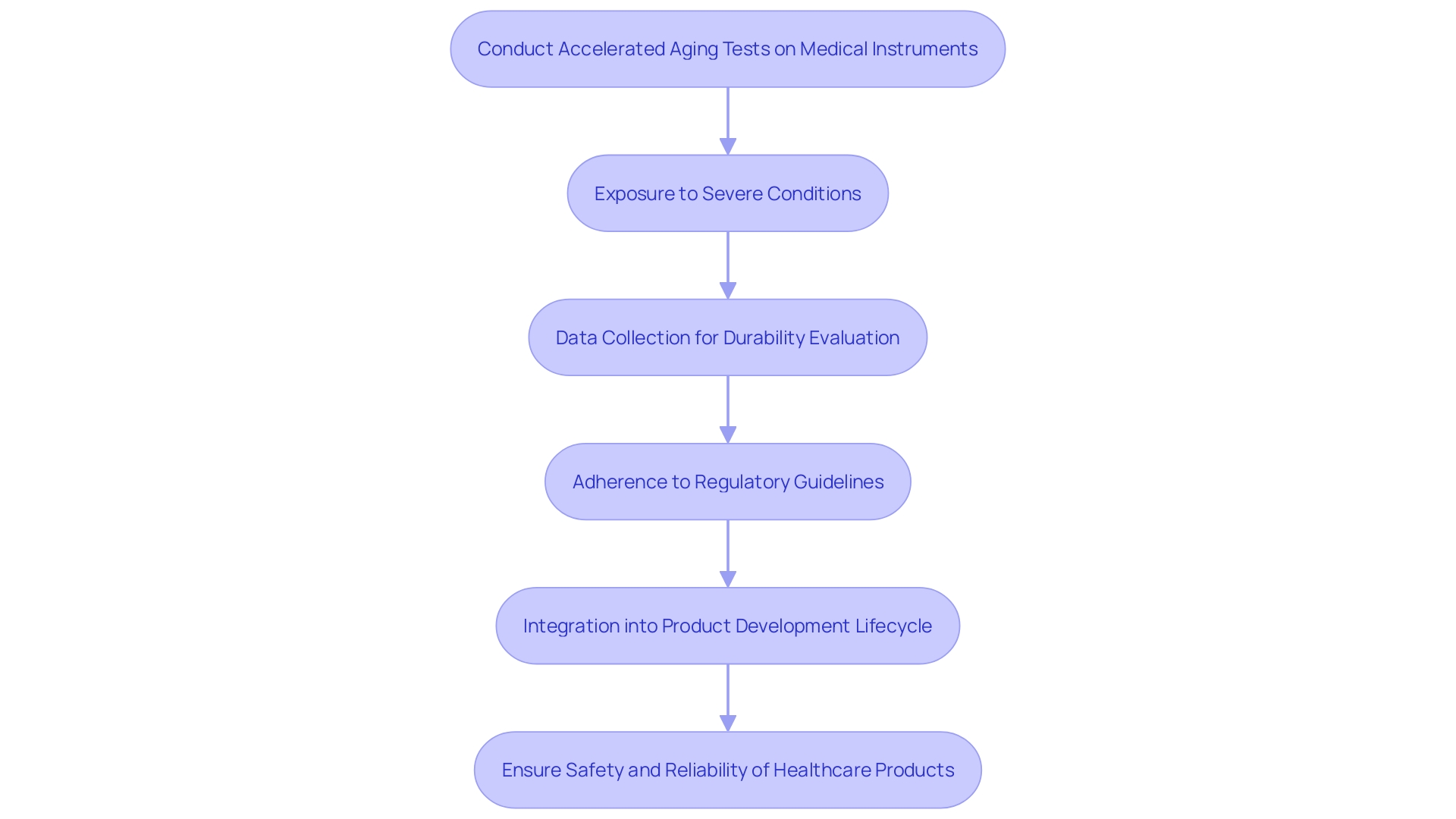
Accelerated aging tests expose medical instruments to severe conditions that mimic the impacts of prolonged use and deterioration. These tests typically involve exposing equipment to elevated temperatures and humidity levels to expedite natural degradation processes. The data collected from these tests is crucial in evaluating the durability and stability of materials, components, and the whole system. This information is essential for ensuring adherence to regulations and effective risk management.
The significance of following guidelines cannot be overstated, as it guarantees the safety and welfare of consumers while reducing potential risks and dangers linked to medical devices. For instance, OECD guidelines like the Conflict Minerals policy, which aims to prevent companies from contributing to conflicts through their sourcing practices, highlight the significance of adhering to regulatory standards. This adherence extends to understanding the specific regulations that apply to different markets and ensuring global conformity to avoid significant penalties.
Moreover, the incorporation of regulations into the product development lifecycle demonstrates a commitment to quality, safety, and customer satisfaction. Organizations that keep track of their Bill of Materials (BOM), including component serialization and lot traceability, are better equipped to handle risks related to compliance changes. 'This practice is not just advantageous for meeting regulations but also enhances trust within the industry and among customers.'.
In summary, accelerated aging and shelf life testing provide essential insights into the long-term performance of healthcare apparatus. 'These tests, along with strict adherence to regulatory standards, guarantee the production of safe, reliable, and compliant healthcare products, ultimately benefiting both manufacturers and end-users.'.
The Role of ASTM F1980 in Accelerated Aging
ASTM F1980 is a crucial standard guide that outlines the procedures and requirements necessary for the accelerated aging testing of healthcare instruments. This standard is instrumental in establishing a robust framework for conducting tests that yield reproducible and reliable results, ensuring that medical devices meet safety and efficacy standards under expedited conditions.
By adhering to ASTM F1980, manufacturers can significantly bolster the credibility of their shelf life claims. This adherence is not just a matter of rule compliance but also a step towards enhancing the trust and reliability of their products in the market. The implementation of ASTM F1980 facilitates the recognition and acceptance of testing methodologies by regulatory bodies, providing a clear pathway for market approval and consumer confidence.
In the constantly changing environment of healthcare equipment regulation, the significance of trustworthy and uniform evaluation cannot be exaggerated. 'Real-time aging studies, such as those outlined in ASTM F1980, are crucial for accurately predicting the longevity and stability of medical instruments.'. These studies provide essential data that supports compliance and market readiness, ensuring that devices perform safely and effectively over time.
'The role of consensus standards, like ASTM F1980, in governance frameworks further underscores their value.'. Created through strict consensus principles, these standards enhance quality by ensuring transparency, stakeholder participation, and due process. 'This thorough conformity evaluation is essential for upholding a robust governance structure, encouraging innovation, and standardizing technologies that enable patient access to new instruments.'.
Grasping and applying ASTM F1980 enables producers to maneuver through the intricacies of regulatory adherence effectively, reducing setbacks and mistakes in the market introduction of healthcare products. As the industry continues to evolve, staying informed about such standards remains essential for advancing healthcare technology and improving patient outcomes.
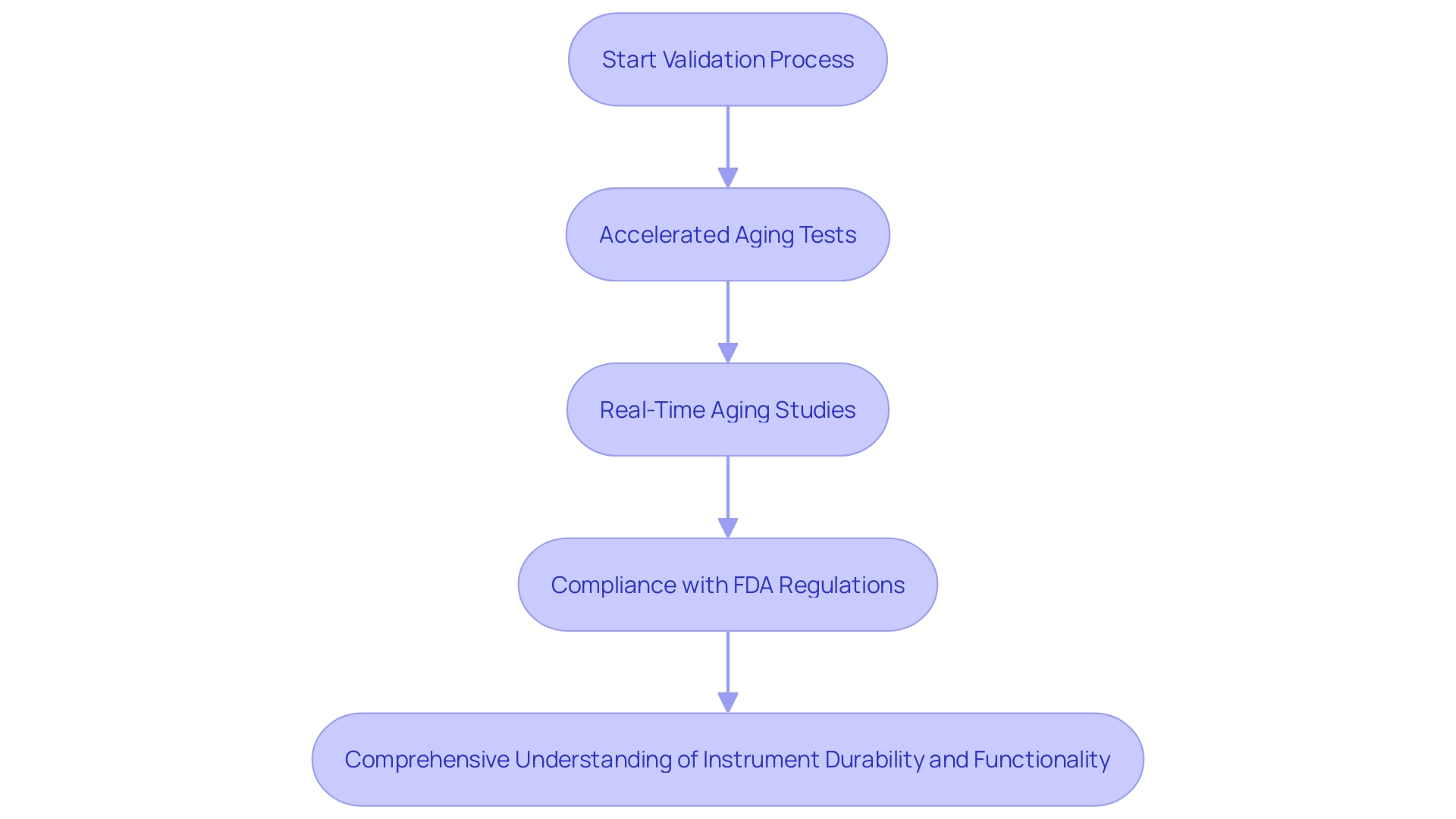
Combining Accelerated Aging with Real-Time Aging Studies
Accelerated aging tests provide essential understanding of the durability and functionality of medical instruments. However, these tests must be complemented by real-time aging studies to ensure comprehensive validation. Real-time aging involves monitoring instruments over their intended shelf life under standard storage conditions. This combination is vital for manufacturers, as highlighted by recent regulatory demands set by the FDA, which emphasize the importance of validating accelerated aging results. By integrating both accelerated and real-time aging data, manufacturers can ensure their predictions are accurate and reflective of actual device performance over time, ultimately securing compliance and enhancing patient safety.
Conclusion
Accelerated aging testing is essential for the medical device industry, enabling manufacturers to accurately estimate the shelf life and reliability of their products under stress conditions. Through controlled simulations, the performance of devices can be projected over extended periods, ensuring safety and efficacy. This process is critical not only for regulatory compliance but also for enhancing health outcomes for patients.
The integration of robust testing methodologies, alongside advancements in digital technologies like AI and machine learning, further amplifies the precision of accelerated aging tests. As the regulatory landscape evolves, particularly with the introduction of guidelines focused on AI, manufacturers must remain vigilant to maintain compliance and competitiveness in the market. The importance of understanding and adhering to standards, such as ASTM F1980, cannot be overstated, as they provide a framework for reliable testing and bolster the credibility of shelf life claims.
Moreover, the combination of accelerated aging with real-time aging studies offers a comprehensive validation approach. Such integration ensures that predictions regarding device performance are accurate and reflective of actual conditions, thereby securing regulatory compliance and enhancing patient safety. In summary, the meticulous application of accelerated aging tests, coupled with adherence to regulatory standards, is vital for the development of safe, effective, and reliable medical devices that ultimately benefit both manufacturers and end-users.




