Introduction
In the realm of medical technology, Contract Research Organizations (CROs) play a pivotal role in driving healthcare innovation forward. These entities provide a range of indispensable services, from concept generation to regulatory compliance, that accelerate the development of medical devices. Collaboration with a CRO grants access to their expertise, resources, and networks, all crucial in bringing new medical solutions to market efficiently.
In this article, we will explore the benefits of partnering with CROs, successful collaborations in the medtech sector, challenges and solutions in CRO collaborations, and future trends and innovations in the field of medical device CROs. Stay tuned to discover how these partnerships are shaping the evolution of medical devices and improving patient outcomes.
The Role of Medical Device CROs in Healthcare Innovation
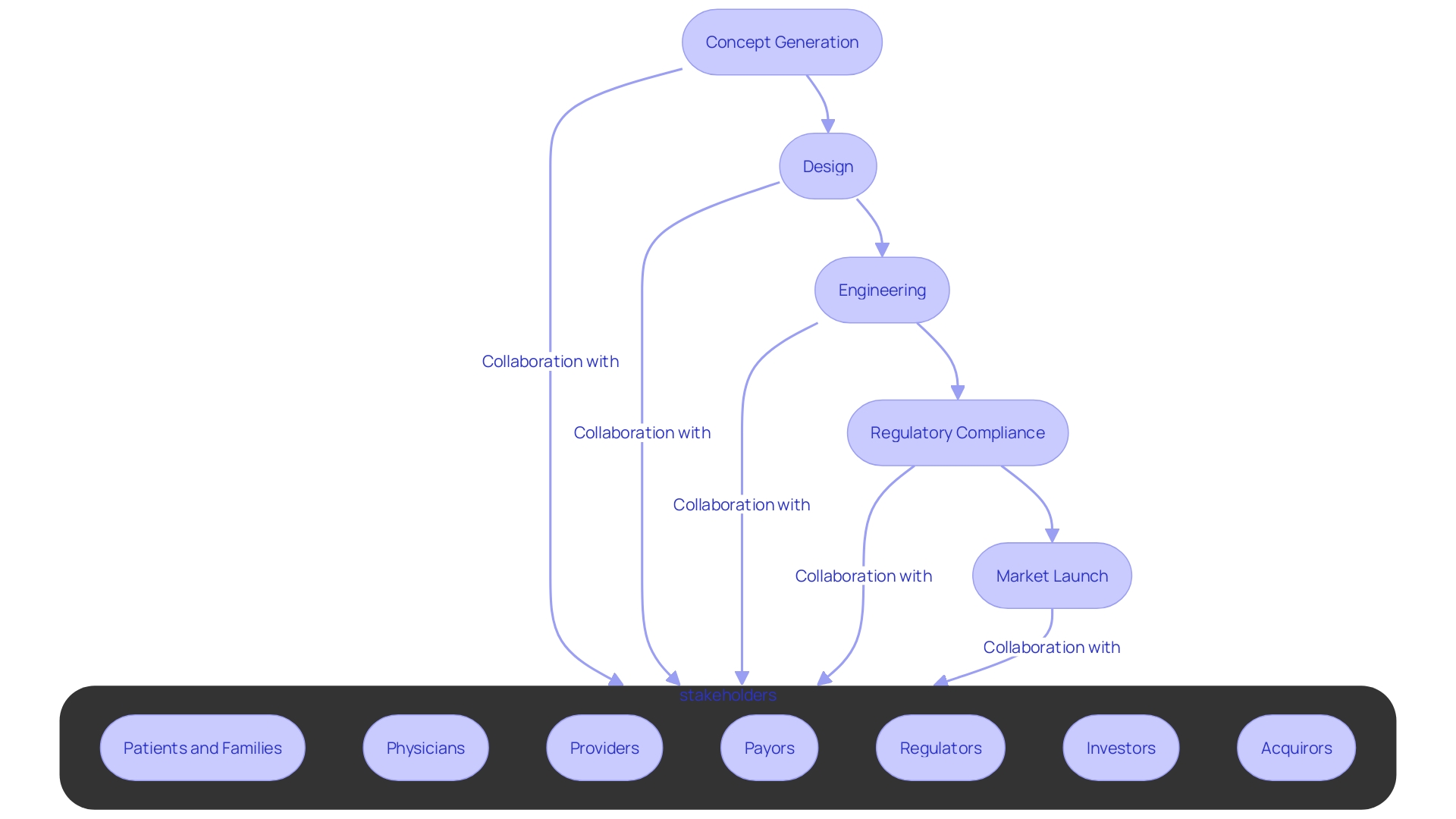
In the realm of medical technology, Contract Research Organizations (CROs) are pivotal in propelling healthcare innovation forward. These entities are adept at extending a host of indispensable services that bolster medical device development, encompassing concept generation, design, engineering, and ensuring meticulous regulatory compliance. Collaboration with a CRO means accessing their profound expertise, state-of-the-art resources, and extensive networks, all of which are critical for hastening the innovation cycle and bringing new medical solutions to market swiftly and efficiently.
Medical device development is an intricate process that requires a seamless melding of multiple stages, from the initial conception to the final market introduction. Firms that orchestrate the development of medical technology seek partners who can navigate this complexity. A comprehensive service suite from a CRO not only secures a fluid transition through these stages but also conserves resources and amplifies the success rate of the project.
Dr. Thomas Fogarty, a renowned innovator in the medical field, puts it succinctly: "An idea, by itself, has no importance whatsoever; it is the implementation of that idea and its acceptance by others that brings benefit to our patients." CROss embody this principle by integrating cutting-edge technologies with keen market insights, ensuring that new medical devices not only meet stringent clinical requirements but also fulfill the nuanced demands of the healthcare ecosystem. By doing so, CROss provide an invaluable bridge between the concept and the clinic, enhancing patient outcomes and advancing medical science.
Case Study: Successful Collaboration between Medical Device Companies and CROs
In collaborative efforts involving the US and Latin America, particularly within the medtech sector, numerous innovations are emerging. Take, for example, the development of the Cardiac cardiac monitoring device. This breakthrough technology harnesses high-performance sensors and AI algorithms to detect subtle magnetic field variations around the heart, which makes it possible to uncover abnormal cardiac patterns early on.
With its impressive advantages – mobility for point-of-care use, lack of need for special installation, and the ability to operate without additional cooling or shielding – the Cardiac system is a remarkable example of advanced medical imaging technology.
The success of CardiAQ can be attributed to strategic collaborations, such as the one with a Contract Research Organization (CRO) that brought expertise in clinical trials and Regulatory Affairs to the table. This partnership facilitated a smoother navigation through the regulatory landscape, accelerating the device's entry to the market – a critical step given cardiovascular diseases remain the leading global cause of death according to the World Health Organization, with estimates of 17.9 million lives claimed each year. Delivering technologies like CardiAQ to the market is not only about meeting healthcare needs but also about addressing complex legal and regulatory environments, a challenge that's expertly navigated through collaborative innovation.
Leading figures in the medical and innovation community, such as Dr. Thomas Fogarty, emphasize that the true value of an idea lies in its implementation and subsequent acceptance by the healthcare ecosystem. The timeline from concept generation to market launch involves concept generation, design, engineering, and regulatory compliance. As digital healthcare experts from Human have pointed out, collaboration across stakeholders is key: providers, payers, pharma companies, and beyond, all aiming to improve patient outcomes while tackling cost and efficiency.
With over 710,000 patents filed in the medical devices industry over the past three years, as reported by GlobalData, the focus on early diagnosis, reducing patient recovery times, and leveraging technologies such as machine learning and digitalization, is accelerating the evolution of medical devices into the future.
Benefits of Partnering with Medical Device CROs
Collaborating with Contract Research Organizations (CROs) can significantly streamline the medical device development process. These organizations come equipped with a deep understanding of regulatory requirements to ensure compliance with entities like the FDA, which categorizes medical devices from low to high risk. Their familiarity with the guidelines and processes of regulatory authorities guarantees adherence to the necessary protocols, reducing the likelihood of encountering hindrances in the development trajectory.
CROs also extend comprehensive resources such as advanced facilities and specialized machinery that are essential for the execution of high-quality clinical trials and robust data collection. Their endemic networks of seasoned professionals enable the facilitation of intricate development tasks, such as those encountered by Everyplace Labs during the design of their automated diagnostic kiosk, which needed to prevent cross-contamination while running multiple tests concurrently.
From a cost perspective, CRO partnerships can prove to be economically advantageous. By leveraging their established relationships, CROss can acquire materials and services at competitive prices. Additionally, their proficiency in risk management and quality control plays a crucial role in early detection and resolution of potential issues, thus deferring the likelihood of expensive delays and ensuring the smooth progress of device development.
As highlighted by digital healthcare experts like Courtney DeSisto Obecny from Huma, collaboration across stakeholders, which includes patients, providers, and pharma companies, is a cornerstone in enhancing healthcare delivery. This collective effort is salient not only in reducing costs and improving efficiency but also in ensuring better patient care outcomes—goals that are mirrored in the CRO partnership model.
In terms of return on investment, businesses like Human notice the trend towards diverse medical device exit strategies such as acquisitions, IPOs, licensing, partnerships, and strategic alliances, as outlined by healthcare business development veterans with decades of experience. These avenues provide tangible benefits to startup medical device companies, while also potentially reducing the costs associated with bringing a new product to the market.
Ultimately, the decision to integrate services with a CRO should consider their ability to navigate the complex landscape of medical device regulation and market dynamics, thereby improving the success rate of innovative medical instruments and solutions like wearable technologies and Sand, which are at the forefront of medical device evolution.
Challenges and Solutions in Medical Device CRO Collaborations
Partnering with Contract Research Organizations (CROs) for medical device development encapsulates the complexities and the necessity for stringent adherence to communication, data security, and intellectual property management. The importance of clear communication and alignment cannot be understated; a business development leader with decades in the sector emphasizes the cruciality of setting a project on the right foot with a proper kick-off meeting. There, the priorities of budget, time, and quality are demarcated, providing fertile ground for a flourishing partnership.
As noted by industry experts, medical technology today is on the cusp of a paradigm shift, where prevention and interception will take precedence over cures. This shift necessitates a harmonious blend of innovation, such as the pivotal role of pulse oximetry in COVID-19 management, with regulation, particularly as medical devices become more integrated with data analytics.
Moreover, the digital transformation of patient communication signifies a broader trend that CROs and medical device companies can tap into—leveraging digital tools not only for therapeutic outcomes but also for compliance and operational excellence. The complexity of meeting regulatory requirements, as highlighted by the participation of nearly two-thirds of medical device companies in e-labeling initiatives, underscores the need for CROss and their partners to align closely on the prerequisites for market readiness and patient safety.
A multi-faceted approach, encompassing regular interactive sessions, contractual agreements protecting sensitive data, and mutual efforts towards streamlining processes with smart and integrated data analytics, are a few measures pivotal for countering the typical hurdles faced in CRO collaborations. Such strategies resonate with the insights shared by stalwarts like Johnson & Johnson Innovation, underscoring that success lies not only in the vision but also in the operational execution within the ever-evolving medtech domain.
Future Trends and Innovations in Medical Device CROs
The medical device industry is witnessing transformative changes as clinical research organizations (CROs) embrace digital health and remote monitoring technologies to enhance clinical trials. Pioneering the shift towards patient-centric methodologies, CROs are integrating wearable devices and telemedicine solutions, allowing for the collection of real-time data, and enabling remote patient monitoring. The implications for clinical trials are profound; such technologies not only streamline patient recruitment and retention but also elevate the quality of data while minimizing the burden on patients and healthcare providers.
Advancements in artificial intelligence (AI) and machine learning are further propelling CROs towards heightened efficiency. AI algorithms are now being deployed to sift through extensive clinical trial data, pinpointing patterns that lead to more accurate and expedited decision-making processes. These developments represent a significant leap in the use of technology within medical device CROs, offering the potential for accelerated healthcare innovation.
However, these advancements come with their own set of challenges. Sam Liu from Vivalink highlights the main issues, as varying device types and operating systems complicate compatibility efforts, and using patients' own devices could introduce security vulnerabilities with personal health information access. Despite these challenges, the familiarity with personal devices leads to better compliance and data collection, according to Liu, who also notes the difference between medical wearables and supplementary consumer devices.
Additionally, the burgeoning field of digital therapeutics, which encompasses software-driven treatments for medical disorders, is also gaining traction. This field, though still in its infancy in clinical trial testing, exemplifies the potential of digital solutions in managing chronic diseases and neurological conditions, albeit with current limitations in insurance coverage and widespread adoption.
When considering medical device development, a comprehensive understanding of the entire process, from concept to market launch, is essential. Finding a development partner with extensive technical expertise, regulatory knowledge, and market insights is critical for a seamless and successful project. This is reflected in the evolving technological landscape, as CROss continue to adapt to and develop new systems and solutions aimed at meeting the dynamic needs of the healthcare industry.
Conclusion
In conclusion, partnering with Contract Research Organizations (CROs) is paramount for driving healthcare innovation and improving patient outcomes. CROs offer expertise, resources, and networks that accelerate medical device development and ensure compliance with regulatory requirements.
Successful collaborations between medical device companies and CROs, like the CardiAQ cardiac monitoring device, demonstrate the strategic benefits of these partnerships. CROs navigate the regulatory landscape effectively, accelerating market entry and addressing complex legal environments.
Partnering with CROs provides several advantages. They have in-depth knowledge of regulatory requirements, reducing development obstacles. CROs offer comprehensive resources, including advanced facilities and specialized machinery, for high-quality clinical trials.
These partnerships also bring economic benefits through established relationships and efficient issue resolution.
Challenges in CRO collaborations, such as communication and data security, can be overcome through clear alignment and the use of digital tools for compliance and operational excellence. Close collaboration and smart data analytics are crucial for successful partnerships.
Future trends in medical device CROs include integrating digital health and remote monitoring technologies, leveraging AI and machine learning for improved decision-making, and the emergence of digital therapeutics for managing chronic diseases. These advancements enhance clinical trials, streamline patient care, and drive innovation.
Overall, partnering with CROs streamlines medical device development, provides access to resources, and navigates regulatory complexities. As the healthcare industry evolves, CROs adapt to meet its dynamic needs, shaping the future of medical devices and benefiting patients worldwide.




