Introduction
Medical device innovation is a complex process that requires expertise in various areas, from concept generation to market launch. Consulting firms play a crucial role in guiding companies through this journey, providing the necessary knowledge and support to navigate challenges and ensure success. In this article, we will explore the importance of consulting firms in medical device development, their role in addressing key challenges, and the benefits of collaborating with them.
By understanding the value they bring to the table, medical device companies can make informed decisions and optimize their innovation initiatives.
Case Study Overview
Medical device innovation is a comprehensive endeavor that extends from the initial concept generation through to design, engineering, regulatory compliance, and ultimately market launch. The role of consulting firms in this domain is pivotal, as they provide the expertise and guidance necessary to navigate the intricacies of the development process. Covering a full spectrum of services, these firms enable a seamless transition between project phases, saving both time and money while bolstering the likelihood of success.
A successful medical device development partner is characterized not only by their technical acumen but also by a profound grasp of the regulatory environment and market trends. This expertise is crucial in ensuring that medical devices meet the complex needs and preferences of various users, including patients, clinicians, and hospital staff. The approach of user-centered design, with a particular emphasis on patient-centered solutions, is integral to the creation of medical devices that offer meaningful and impactful experiences to all stakeholders involved.
In the rapidly evolving landscape of healthcare, private practices and companies are increasingly leveraging digital platforms to streamline operations and enhance patient care. Such technological advancements underscore the importance of service design in medical device innovation, ensuring that every touchpoint aligns with the user's requirements.
Medical device consultants, whether operating independently or within a firm, offer a comprehensive range of services. From conducting relevant literature research to advising on regulatory compliance pathways, these professionals are instrumental in guiding manufacturers through the entire lifecycle of medical device development. As the healthcare field swiftly adapts to digitalization, the expertise of medical device consultants becomes even more critical in maintaining a competitive edge.
In light of these considerations, selecting an adept medical device development company is a decision of paramount importance. The best path forward involves a partner that demonstrates a broad range of capabilities and a deep understanding of the medical device landscape, as echoed by industry leaders and buyer's guides. Such collaborations are designed to ensure successful outcomes, whether it be through achieving regulatory compliance, enhancing product quality, or facilitating a profitable exit strategy for medical device startups.
Identifying Key Challenges in Medical Device Development
Navigating the complexities of medical device innovation involves understanding and addressing several pivotal challenges. Medical device companies are tasked with complying with stringent regulatory standards, like the Medical Device Regulation (MDR) in Europe, which necessitates careful navigation for certification in the market. This is further compounded by the need to incorporate cutting-edge technology in product development while maintaining cost-efficiency and staying ahead in a competitive market landscape.
These challenges underscore the importance of in-depth knowledge in various disciplines ranging from software development to optical design. For instance, developers must have a thorough comprehension of medical devices before developing software, particularly considering the security aspects required to comply with regulations. Cryptographic communication protocols, such as Transport Layer Security (TLS), are essential in ensuring secure and encrypted communication with medical devices.
Moreover, the medical device sector is marked by a remarkable diversity in products, from basic consumer items like spectacles to highly complex systems such as MRI machines and pacemakers. This diversity spans across human factors and device factors, requiring a comprehensive understanding of individual differences and multidisciplinary approaches. Given the World Health Organization cites over 10,000 types of medical devices available, the scope of innovation and the need for effective management strategies are vast.
To effectively maneuver in this multifaceted environment, companies can benefit from the insights provided by experienced professionals in the healthcare sector. Leaders who have navigated the path to successful exits—whether through acquisition, IPO, licensing, or strategic alliances—share traits such as grit and perseverance, coupled with an acute understanding of business factors that drive success.
The medical devices market, projected to generate significant revenue globally, is driven by the growing demand for innovative healthcare solutions. Market segmentation reveals an extensive array of products essential for patient care and healthcare delivery, with ongoing growth influenced by demographic shifts, the prevalence of chronic diseases, and enhancements in healthcare infrastructure. As such, the role of consulting firms in bridging the gap between innovation and successful market entry becomes increasingly pivotal for medical device companies.
Role of Consulting Firms in Addressing Challenges
Medical device companies are at the forefront of healthcare innovation, dealing with a plethora of challenges ranging from regulatory compliance to the integration of advanced technologies. Consulting firms are pivotal in navigating these complexities, leveraging their deep industry insight and proficiency in cutting-edge strategies to assist these companies in achieving their objectives effectively.
Consulting services are instrumental in bridging the gap between various disciplines within medical device development. A thorough analysis using first principles or optical design software is often crucial to understand the key variables and optimize success chances. This multidisciplinary approach ensures that optics, a core component of many medical devices, is not treated in isolation but as part of a larger system encompassing electrical, mechanical, and software development considerations.
The role of consulting firms extends to the foundational steps of medical device projects. In the initial stages, they emphasize the importance of aligning all stakeholders, including external partners, to establish and prioritize the project's key elements such as budget, time, and quality. This alignment is essential for setting a clear direction and ensuring that everyone involved has a common understanding of the project's objectives and expectations.
The diversity of medical devices, from simple consumer products to complex diagnostic systems, requires a vast knowledge base that extends beyond pharmaceuticals to include materials science, bioengineering, and information technology, among other fields. Consulting firms possess the technical expertise and regulatory knowledge to support medical device companies across this broad spectrum.
In light of the rapidly evolving healthcare landscape, where many independent practices are being acquired, consulting firms also advocate for the adoption of digital solutions such as virtual care platforms. These innovations can streamline operations and enable private practices to maintain their competitiveness by focusing on delivering exceptional patient care.
Successful exits for medical device companies, such as acquisitions, IPOs, licensing agreements, or strategic alliances, are often facilitated by the strategic planning and industry acumen of consulting firms. These firms provide a wealth of services including research, product design, regulatory guidance, and marketing strategies, which are critical for companies to navigate the complex journey from concept to market.
In summary, consulting firms offer an array of services that are integral to the success of medical device companies. They are not only advisors but also strategic partners that contribute to the development of groundbreaking medical technologies, ensuring these innovations reach the market efficiently and continue to advance patient care.
Consulting Firm Services: Advisory and Support
Medical device companies navigate through complex innovation pathways, from concept inception to healthcare implementation. Consulting firms are pivotal in guiding these companies, offering a comprehensive range of services that cater to the intricate demands of device development and market introduction. These services notably encompass strategic planning, regulatory advising, market research, product development navigation, and project management aid, each tailored to the multifaceted aspects of the industry.
Service design is integral to creating medical devices, focusing on those who influence patient care, such as clinicians, nurses, and hospital staff. This approach ensures that all end-users' unique needs and experiences are considered, which is vital for user-centered design. User research, usability testing, and iterative design processes are employed to enhance how users interact with medical devices, improve individual experiences, and ensure products effectively meet users' needs.
The MedTech consultancy Archetype exemplifies this commitment by supporting innovators in securing market approval for medical devices. With an extensive network of experts and a comprehensive suite of services, Archetype embeds market approval goals into every development phase, from concept to product design to regulatory documentation.
Innovations like the CardiAQ system demonstrate the industry's advancement, with high-performance sensors and AI algorithms that improve early detection of diseases through accessible medical imaging. The development of such technology requires a synergy of technical expertise, awareness of regulatory frameworks, and an understanding of market dynamics, which consulting firms provide.
Successful medical device projects often begin with a clear vision of the end-user impact and the value proposition. This strategic foresight, combined with attention to the project's priorities, sets the stage for a successful journey from initial team meetings to market launch. Consulting firms offer invaluable support in aligning medical device innovation with these crucial business and development considerations.
Case Study: Successful Collaboration Between Medical Device Company and Consulting Firm
Exploring the collaboration between a medical device company and a consulting firm unveils the intricate process of developing solutions that cater not only to patients but also to the entire ecosystem of healthcare providers. The user-centered design approach, which was instrumental in this partnership, emphasizes the creation of medical devices by considering the diverse experiences and needs of various users, including clinicians, caregivers, and hospital support staff.
User-centered design in medical devices goes beyond the individual patient experience. It involves a deep dive into the behaviors, motivations, and needs of all stakeholders who interact with the device. This method ensures that medical devices are not only effective for patients but also enhance the workflows of healthcare professionals, supporting staff, and technicians.
The iterative design process, underpinned by user research and usability testing, is pivotal in refining these devices to meet the exacting demands of the healthcare environment.
The successful launch of the innovative medical device hinged on this holistic design philosophy. The consulting firm's expertise in service design played a critical role, ensuring the device met the multifaceted requirements of a real-world healthcare setting. The recent introduction of the Cardiac system, with its advanced sensors and AI algorithms, is a testament to the effectiveness of such an approach.
The system's mobility, ease of use, and rapid measurement process exemplify how user-centered design can lead to medical devices that are not just innovative but also practical and accessible in a clinical context.
Highlighting the significance of selecting the right development partner, the collaboration focused on a full spectrum of services, from concept generation to market launch, ensuring a seamless transition through each phase. The consulting firm's comprehensive understanding of regulatory compliance and market dynamics was crucial to the project's success. This partnership underscores the importance of a meticulous approach to medical device development, where the synergy between technical expertise and user-centric design culminates in breakthroughs that advance patient care and support the medical community.
Benefits and Outcomes of the Collaboration
The synergy of the medical device company with the consultancy Archetype has led to a series of tangible improvements in the device development lifecycle. By integrating user-centered design, the consultancy has emphasized the importance of considering all stakeholders in healthcare, from clinicians to support staff, in the design process. This holistic approach to design is not only about fulfilling patients' needs but also ensuring that devices are optimized for those who deliver care and maintain healthcare operations.
Archetype's comprehensive suite of services reflects a deep understanding of the entire product development journey, from concept to market launch. They provide expertise in areas such as quality assurance and regulatory documentation, which are critical to navigating the complex healthcare regulations and achieving market approval efficiently. With the medtech industry facing a high failure rate for innovations reaching the market, Archetype's mission to streamline the approval process is valuable, as it can significantly expedite the availability of life-changing medical devices to patients.
Furthermore, the consultancy's approach to service design — studying user behaviors, needs, and motivations — ensures that every interaction with the medical device is impactful and relevant. This is exemplified by the Cardiac system, where advanced sensors and AI algorithms work in tandem to deliver precise medical imaging, thereby facilitating early disease detection and treatment. The mobility and ease of use of the Cardiac system is a testament to the success of user-centered design principles in creating medical devices that are practical and beneficial in a clinical setting.
The collaboration has also leveraged the experience of Dr. Stuart Grant and his network of experts, who possess a wealth of knowledge across various medtech sectors. This collective expertise is a tremendous asset for ensuring that devices not only meet clinical needs but also comply with regulatory standards and are designed with the market in mind.
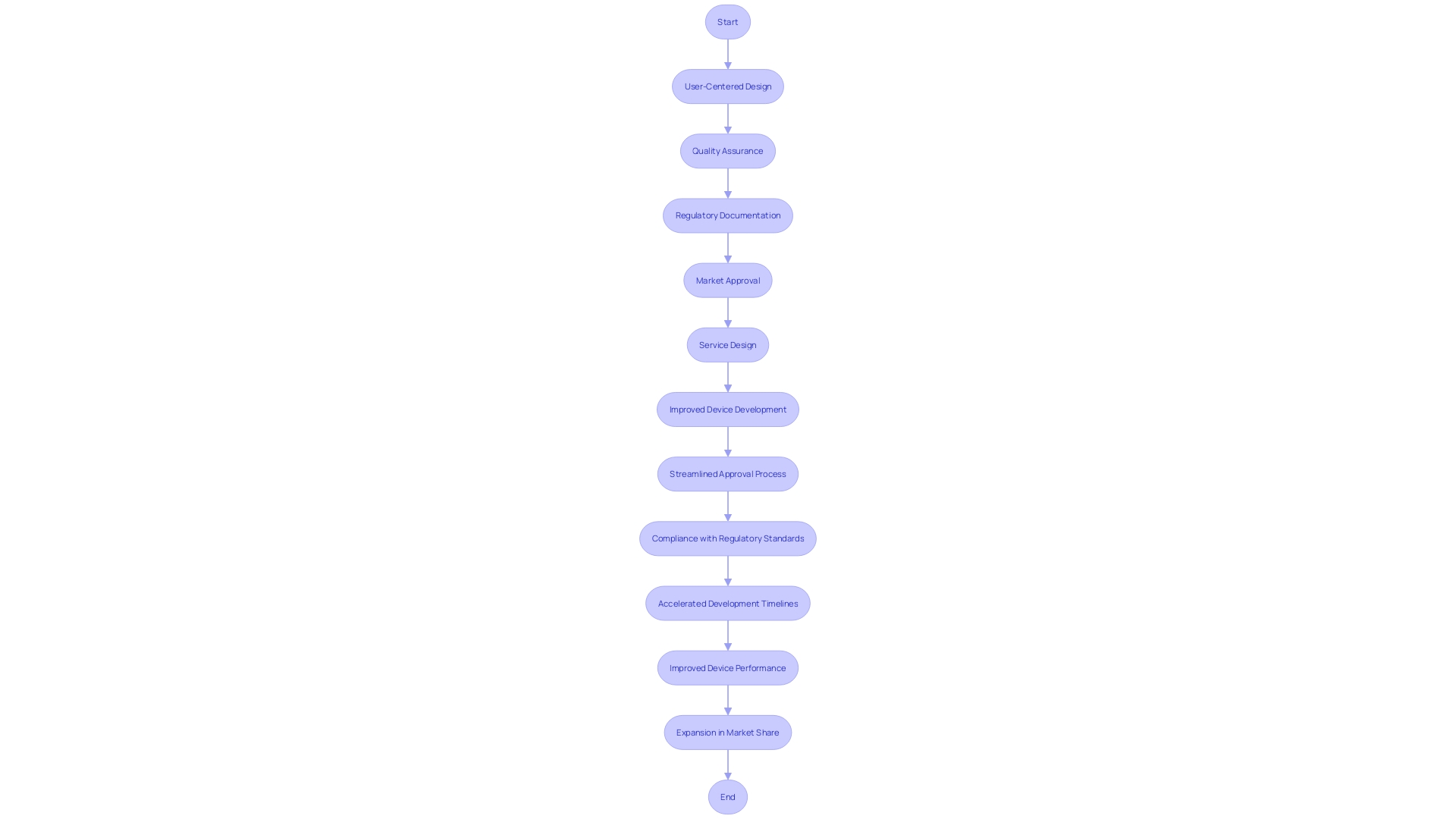
In summary, the partnership has led to accelerated development timelines, adherence to regulatory requirements, strategic market entry, improved device performance, and an expansion in market share. These outcomes highlight the importance of selecting a medical device development partner that can provide a wide array of services and has a proven track record of guiding innovators through the intricate process of bringing medical devices to market.

Best Practices for Engaging Consulting Firms in Medical Device Innovation
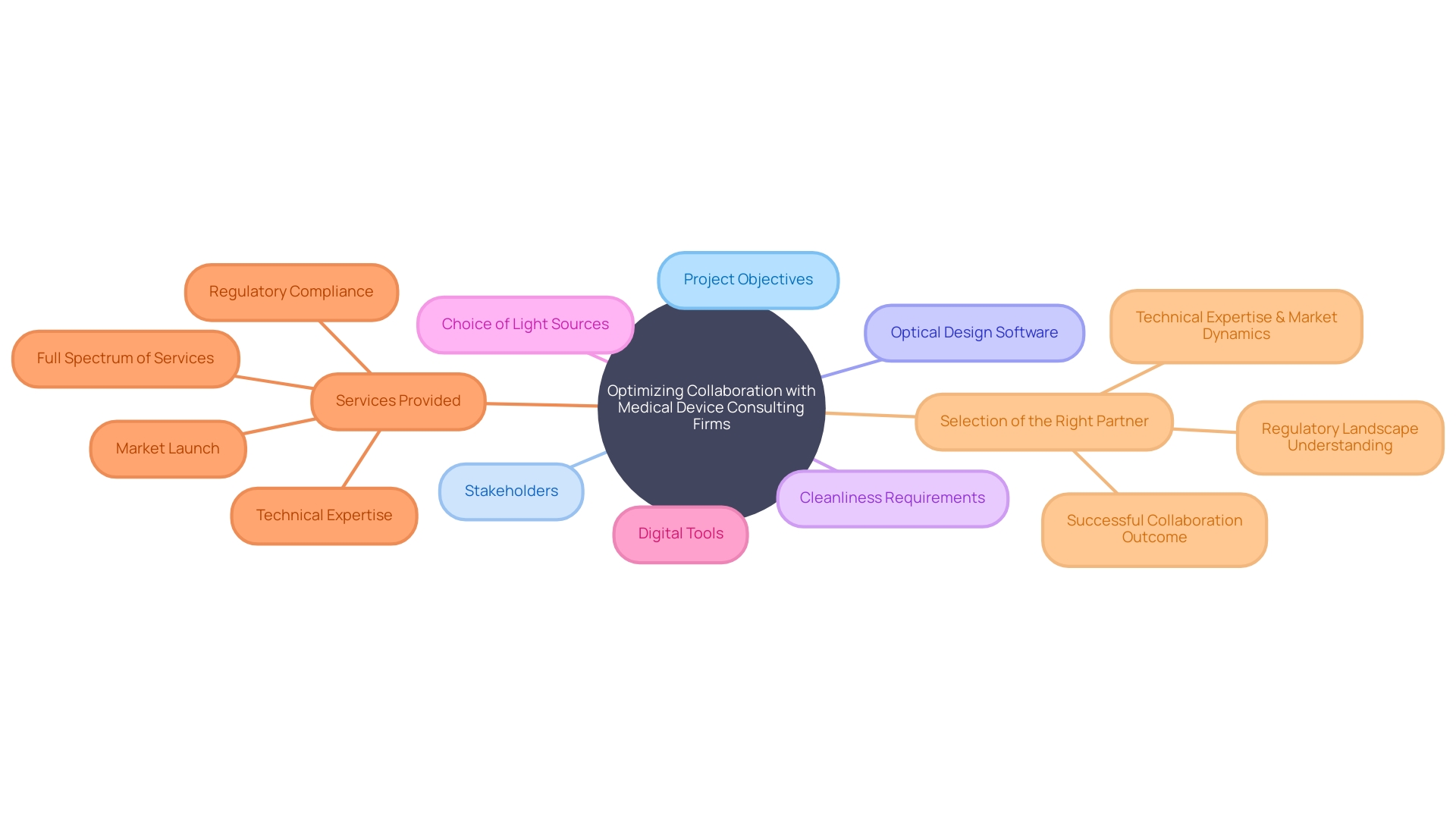
Optimizing collaboration with medical device consulting firms is paramount in the field of medical device innovation. To navigate the intricacies of product development, it's crucial to acknowledge that optics, as an integral component, intersects with various disciplines. Successful collaboration is facilitated by recognizing the systemic nature of the challenges, as underscored by the necessity for comprehensive analysis using first principles or optical design software.
Stakeholders must prioritize project objectives, aligning them with the most critical aspects: budget, timeline, and quality, which are tailored to the unique demands of each project. Emphasizing the initial team meeting sets the tone for a project, ensuring all parties, including external stakeholders, are in sync regarding the project charter. Cleanliness requirements for optical systems, while stringent, should reflect the development stage and intended application, avoiding unnecessary burdens in the early phases.
The choice of light sources, with their varied properties and safety implications, is another cornerstone of medical device development.
As the medical industry evolves rapidly with technological advancements, private practices are leveraging digital tools, such as virtual care platforms, to streamline operations and enhance patient care. Medical device consultants are at the forefront of this transformation, offering a spectrum of services from research and design to regulatory guidance and market strategy, ensuring a device's journey from conception to market is well-informed and compliant.
The expertise of a medical device design partner is a critical determinant of a project's success. A full-service partner provides an integrated approach from concept generation to market launch, ensuring a smooth transition through the design, engineering, and regulatory compliance stages. This holistic approach can result in significant time and cost savings, as well as an enhanced outcome.
In the highly regulated domain of medical device design, the selection of the right partner is essential. Prospects should seek companies with a demonstrated breadth of expertise, ensuring that the chosen collaborator can navigate the regulatory landscape and market dynamics effectively. Such strategic alliances are often pivotal to a successful venture, as evidenced by various successful exit strategies, including acquisitions, IPOs, licensing agreements, and partnerships, which can provide substantial financial returns and add enduring value to the medical device industry.
Next Steps for Medical Device Companies Considering Consulting Firms
When embarking on innovation initiatives, medical device companies must carefully select consulting firms to ensure the success of their projects. The process begins with a rigorous evaluation to find a consulting partner with a proven track record. This involves analyzing the firm's ability to integrate a systems approach, recognizing that optics, electrical engineering, software development, and patient-device interaction are integral to medical device development.
The initial team meeting is a critical juncture where all stakeholders must align on project priorities, which typically encompass budget, timeline, and quality. By establishing a clear hierarchy of these priorities early on, companies can streamline the project's direction and focus.
Choosing the right development partner is vital in the complex, highly regulated medical device industry. Key factors include the partner's comprehensive service range—from concept generation to market launch—and deep expertise in technical, regulatory, and market aspects. It's essential to partner with a firm that offers a seamless transition between development phases, saving valuable time and resources.
Industry experts emphasize the importance of collaboration among various stakeholders to drive healthcare transformation. This synergy is crucial for tackling challenges like treating complex diseases, cutting costs, and enhancing efficiency to ultimately improve patient outcomes. Digital health solutions like wearable technology and Software as a Medical Device (SaMD) are at the forefront of this revolution, offering new possibilities for patient monitoring and treatment.
In conclusion, the selection of a consulting firm is a strategic decision that can significantly influence the trajectory and success of a medical device company's innovation efforts. A partnership built on mutual understanding, expertise, and collaboration is the cornerstone of long-term achievement in the dynamic field of medical device innovation.
Conclusion
Consulting firms play a crucial role in guiding medical device companies through the complex process of innovation. They provide expertise and support, saving time and money while meeting regulatory requirements and market trends. By adopting a user-centered design approach, consulting firms create meaningful experiences for all stakeholders.
Collaborating with consulting firms brings numerous benefits, including accelerated development timelines, adherence to regulations, strategic market entry, improved device performance, and increased market share. To engage consulting firms effectively, medical device companies should prioritize collaboration and recognize the systemic nature of challenges. A strategic approach, from the initial team meeting to market launch, ensures a smooth transition and significant time and cost savings.
Selecting the right consulting partner is essential. Their expertise in navigating the regulatory landscape and market dynamics is crucial for success. A partnership built on mutual understanding, expertise, and collaboration is the cornerstone of long-term achievement in the dynamic field of medical device innovation.




