Introduction
The landscape of medical device research is evolving rapidly, driven by the need for innovative strategies and the integration of digital marketing techniques. In this article, we will explore the importance of these strategies and how they are shaping the future of medical device marketing.
From developing targeted marketing campaigns to enhancing customer experience and leveraging artificial intelligence, we will delve into the key factors that contribute to the success of medical device research and marketing efforts. Additionally, we will discuss the significance of creating detailed buyer personas and optimizing demand generation and lead nurturing. Ultimately, this article aims to provide a comprehensive understanding of the intricacies involved in marketing medical devices and the role it plays in advancing healthcare technology.
The Importance of Innovative Strategies
The exploration of innovative strategies in medical device research is pivotal for the advancement of healthcare technology. These strategies are not a one-size-fits-all solution; they are as diverse as the global markets they aim to serve. A nuanced approach is required, one that considers the unique medical and technological needs of various regions.
For instance, the strategy for a medical device targeting low- and middle-income countries (LMICs) must be tailored to their specific contexts. With over 128 LMICs, including burgeoning economies like China, India, and Brazil, researchers must pinpoint key target markets based on disease incidence and prevalence data. By focusing on a select few representative locations, valuable insights can be drawn despite the inherent differences between countries.
Collaboration is also key. Identifying the right local partners can streamline the process of gathering Voice of the Customer (VoC) research and facilitate stakeholder site visits. The initial team meeting is crucial for aligning stakeholders on project priorities, which typically revolve around budget, time, and quality.
Understanding each team member's role and responsibility, as outlined in the RACI model, lays the groundwork for successful project execution. Furthermore, as Dr. Thomas Fogarty insightfully remarked, the success of a medical device hinges not only on the idea but on its implementation and acceptance within the healthcare ecosystem. This underscores the importance of innovators understanding and addressing the multifaceted needs of patients, physicians, providers, payors, regulators, investors, and acquirors from the outset.
The medical devices industry is witnessing a surge in activity due to the growing need for home care, preventative treatments, early diagnosis, and technologies like machine learning and digitalization. With over 710,000 patents filed and granted in the past three years, it's crucial to recognize that innovations evolve along an S-shaped curve from emergence to maturity. Identifying where an innovation stands in this life cycle is vital for gauging its adoption rate and future impact.
Embracing Digital Marketing for Medical Devices
In the realm of medical devices, which encompass a vast array of apparatus, machines, and software designed for diagnostic and therapeutic purposes, digital marketing has become a cornerstone for promotion and advancement. By leveraging the interconnectedness of digital platforms, researchers and manufacturers can communicate the significance and benefits of their medical innovations to a broader audience.
The strategic use of digital marketing techniques like SEO, social media, and content marketing enables them to highlight the value of medical devices that improve patient care and quality of life. Telehealth, a sector that has seen a remarkable uptake during the recent global health crisis, stands as a testament to the synergy between healthcare and digital innovation.
With teleconsultation and remote monitoring services gaining traction, marketing opportunities in this space have expanded. The digital transformation in healthcare, driven by Big Data, mobile apps, and AI, is reshaping not only patient care but also the distribution of medical supplies and services, making it crucial for medical device marketers to adapt and innovate. As the industry navigates through this digital revolution, it's imperative to connect with the right professionals who can amplify the reach of these medical advancements. For those seeking to navigate the complexities of marketing in the medical device sector, reaching out to a team of experts can streamline the process of identifying the appropriate audience and crafting a message that resonates, ultimately driving forward the mission of enhancing healthcare through technology.

Developing Targeted Marketing Campaigns
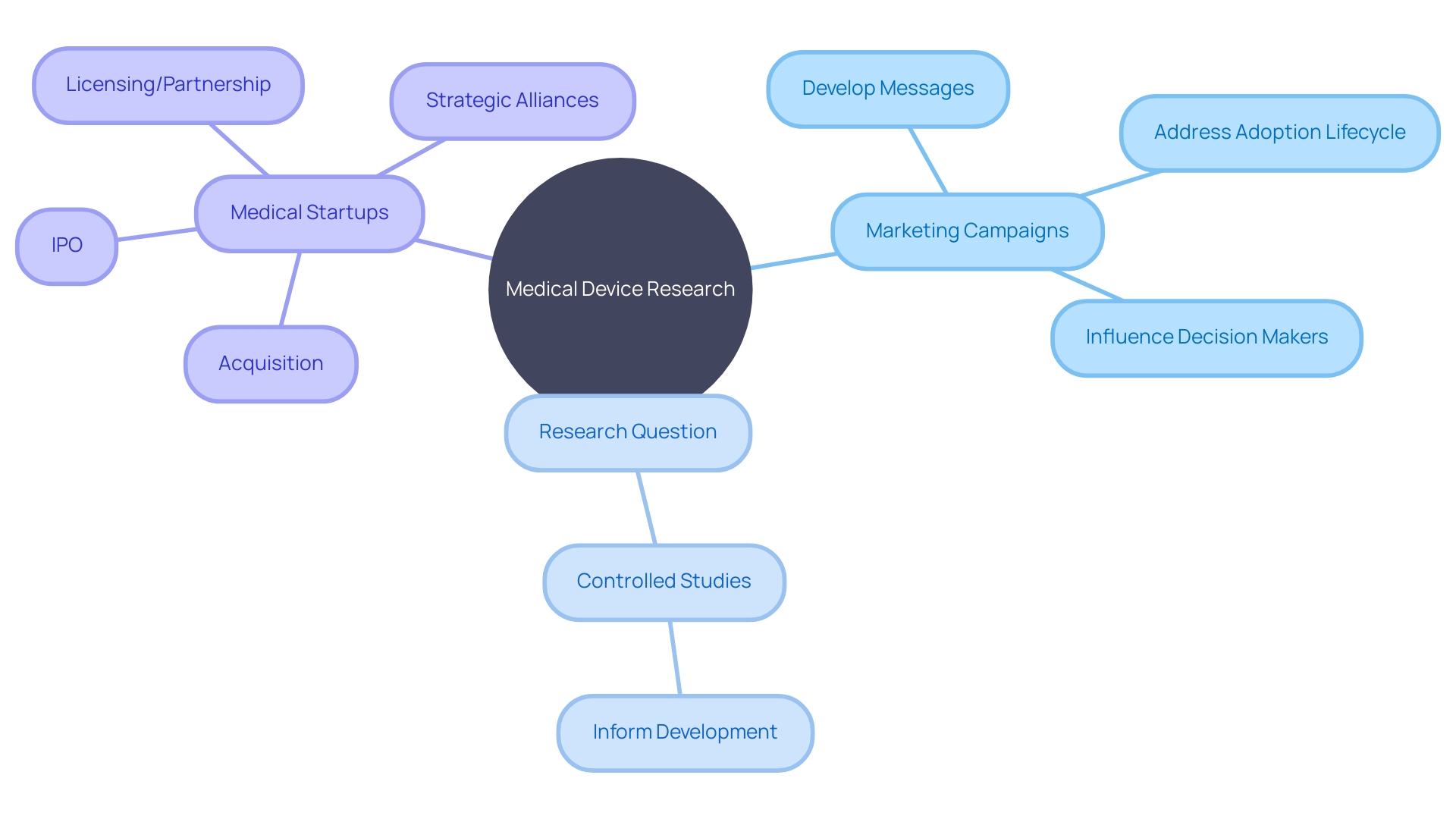
At the core of advancing medical device research lies the strategic alignment of marketing campaigns with the intricacies of controlled medical studies. The initial step is to forge a clear and well-defined research question, which acts as the bedrock for constructing a robust study design.
Researchers must judiciously select variables to manipulate and outcomes to measure, drawing on a meticulously planned experimental design to curtail biases and confounding factors. By paralleling these scientific considerations with marketing efforts, researchers can craft messages that resonate deeply with the medical community, particularly emphasizing how emerging medical technologies—like machine learning, augmented reality, and digitalization—can revolutionize homecare, early diagnosis, and patient recovery.
With over 710,000 patents filed in the medical devices industry in the last three years, as reported by GlobalData, the landscape is ripe with innovations at various stages of the adoption lifecycle. Marketing campaigns must, therefore, be nimble and informed, targeting medical professionals who are attuned to the S-shaped curve of technology adoption—from early emergence to maturity.
As one experienced business development leader notes, the success of medical startups often hinges on grit and perseverance, but also on strategic business moves such as acquisitions, IPOs, or forming alliances. To effectively capture the attention of discerning medical professionals and stakeholders, a proactive approach to public relations is crucial. As highlighted by industry experts, influencing decision-makers requires early and sustained engagement, addressing their risk aversion with well-timed, strategic communications. By integrating these insights into marketing campaigns, medical device researchers can ensure that their innovations are not only groundbreaking but also widely adopted and successful in improving patient outcomes.
Enhancing Customer Experience in Medical Device Marketing
Excellence in medical device research hinges on crafting a user experience that resonates on a personal level with all stakeholders involved in healthcare—from patients to clinicians and maintenance staff. By embracing a holistic approach to design, focusing on the nuances of user-centered and patient-centered strategies, researchers can develop medical devices that not only meet the unique needs of each user group but also create a positive emotional response upon interaction. This approach, rooted in empathy and understanding, leads to greater adoption, improved usability, and ultimately, enhanced clinical outcomes.
Indeed, the emotional interaction a user has when they see or touch a medical device—often occurring instantaneously and before any practical use—can significantly shape perceptions of quality and performance. Addressing this by understanding users' needs and desires through field research and collaboration with Human Factors Engineers is pivotal. It ensures that the design process is informed by real-world experiences and expectations, which is crucial for fostering a sense of confidence and ease among users.
The success of a medical device also extends into the business realm, where strategic decisions can lead to lucrative exits for startups. A combination of grit, perseverance, and strategic business factors—such as acquisitions, IPOs, licensing agreements, and strategic alliances—can culminate in a profitable conclusion of operations or ownership changes. Such outcomes are a testament to the importance of aligning user experience with business acumen in the medical device industry.
The Role of Artificial Intelligence in Medical Device Research
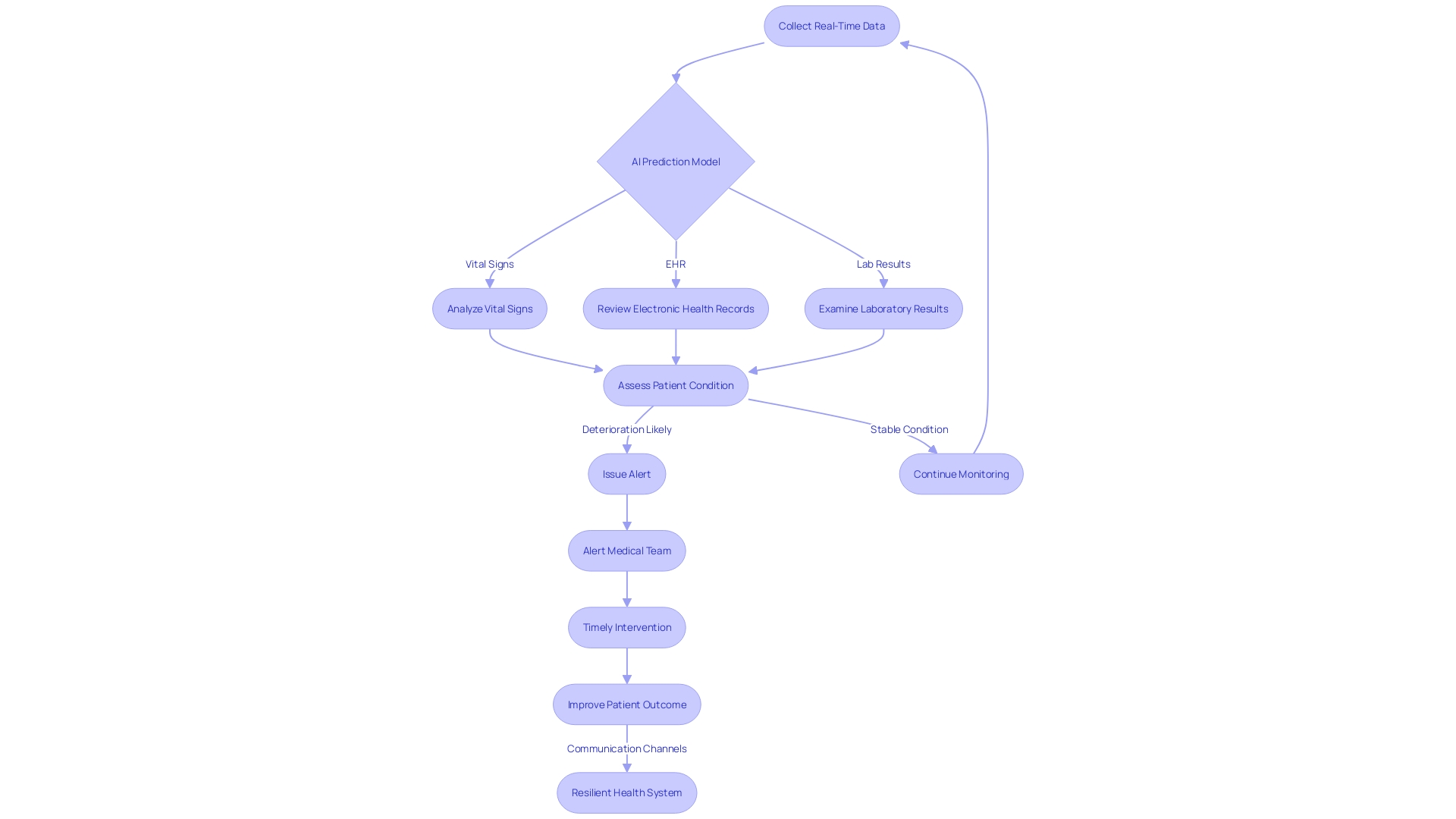
The integration of Artificial Intelligence (AI) into medical device research heralds a new era in healthcare innovation. One exemplary case is an AI-powered prediction model that utilizes real-time data from various sources, such as vital signs, electronic health records, and laboratory results. This model scrutinizes data approximately every 15 minutes to forecast potential patient health deterioration.
In instances where a patient's condition is likely to worsen, the model issues alerts to the medical team, prompting timely interventions and fostering a resilient health system through improved communication channels. In the broader scope of medical device design, AI's role is increasingly pivotal. The design verification phase is crucial, ensuring that the device's performance aligns with its intended design.
This phase involves rigorous testing, as seen with Renishaw's neuroinfuse drug delivery system, which delivers medication directly to the brain, bypassing the blood-brain barrier. Such intricate systems underscore the importance of AI in enhancing device safety and efficacy. Aengus Ó Curraidhín, a senior medical design/development engineer at Renishaw, emphasizes the significance of AI in the future of MedTech.
With Ai's ascendancy, the industry is poised to better manage, diagnose, and treat medical conditions. However, these advancements necessitate robust design and validation processes to guarantee patient safety. As the industry trends towards integrating AI, the thematic intelligence report by GlobalData outlines the technological, macroeconomic, regulatory, and industry trends that are shaping the AI in medical theme over the next 12 to 24 months, highlighting the transformative potential of AI across the medical value chain.
Creating Detailed Buyer Personas for Medical Device Marketing
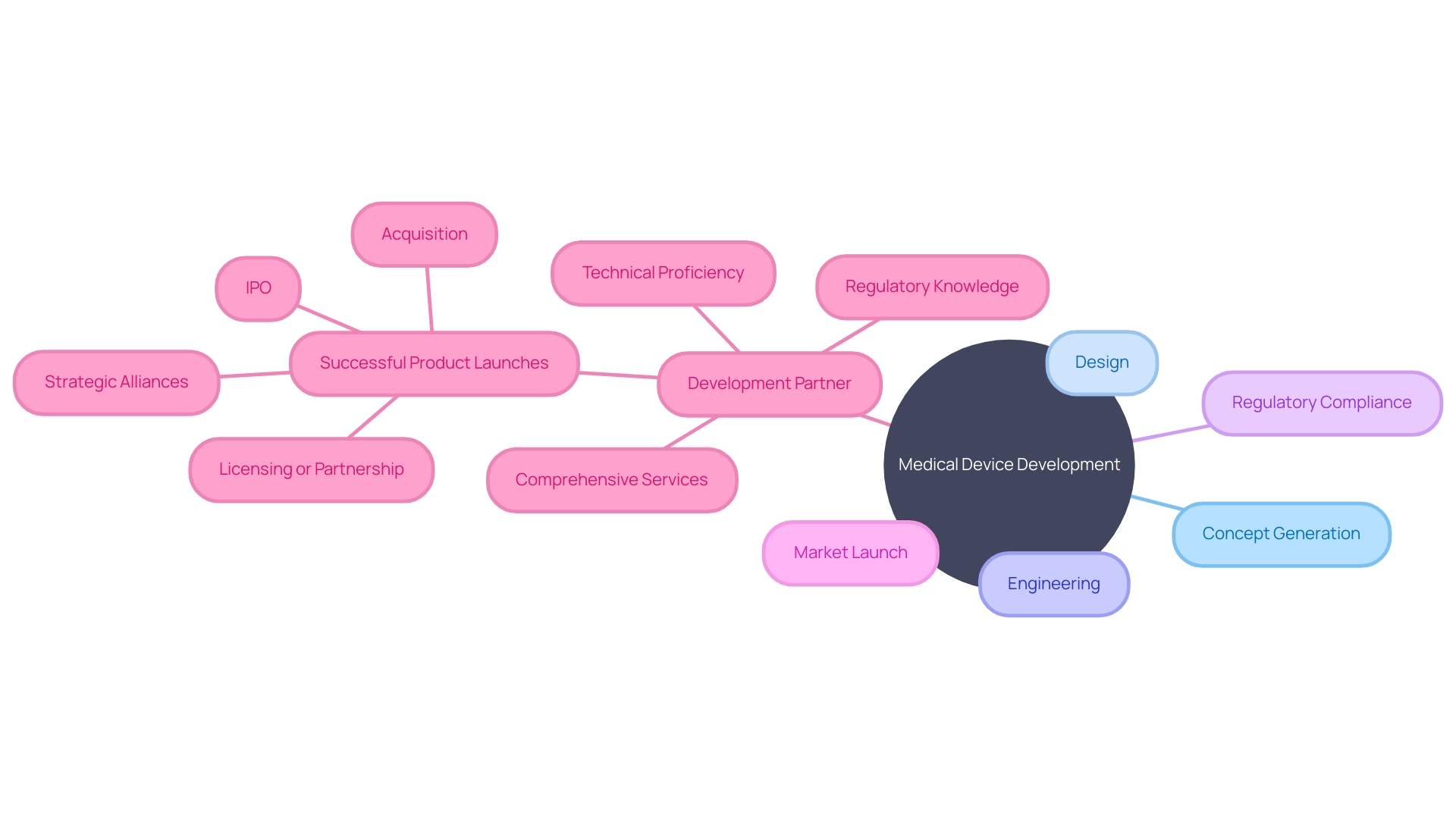
The landscape of medical device development is intricate and multifaceted, encompassing concept generation, design, engineering, regulatory compliance, and successful market launch. Stakeholders in the medical technology space prioritize finding a development partner that offers a comprehensive suite of services. This holistic approach not only streamlines the transition between development phases but also mitigates costs and accelerates the time-to-market, which is imperative for the success of any medical device.
The ideal partner is one that merges technical proficiency with an in-depth grasp of the regulatory environment and market tendencies. Indeed, the World Health Organization (WHO) defines medical devices broadly, ranging from simple tongue depressors to complex diagnostic software, each with its unique set of development challenges. Furthermore, the integration of software with medical hardware is becoming increasingly vital, especially in the digital health domain, providing a substantial competitive advantage.
According to industry experts, a company's history of successful product launches is indicative of its capability to navigate the intricate journey from an idea to market presence. This proficiency is a critical factor for potential buyers and should be a primary consideration. As the medical devices industry evolves, staying informed and making strategic connections with the right development partners is more crucial than ever, especially for stakeholders aiming to navigate the competitive and regulated medtech landscape.
Optimizing Demand Generation and Lead Nurturing
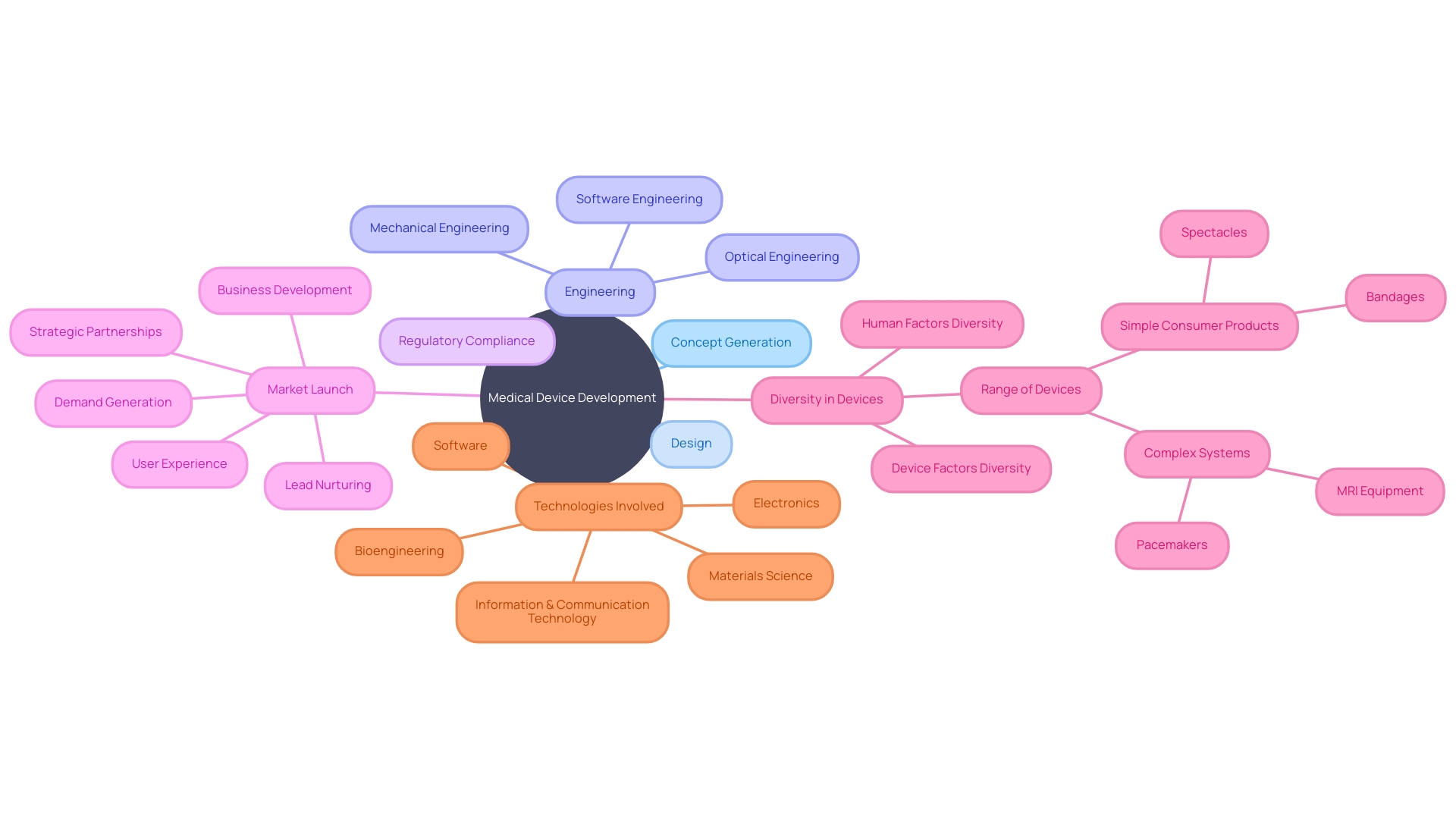
In the dynamic realm of medical device research, the journey from concept generation to market launch is intricate and multifaceted, involving a diverse array of technologies and disciplines. As the healthcare landscape evolves, so too does the complexity of medical devices, ranging from the simplicity of spectacles to the sophistication of MRI equipment and pacemakers.
These devices are not only varied in function but also in the human and device factors they encompass, catering to a wide spectrum of individual differences and needs. To navigate this diversity and complexity, researchers must adopt a holistic approach to development, integrating optical, mechanical, and software engineering with user experience.
This systems-oriented perspective ensures that all aspects of the device's lifecycle are considered, from initial design to patient interaction. With over 10,000 types of medical devices identified by the World Health Organization, the significance of a comprehensive development strategy cannot be overstated.
In this context, demand generation and lead nurturing transcend mere marketing tactics; they become instrumental in bridging the gap between innovation and implementation. By engaging potential users early and guiding them through the decision-making process with valuable information and trust-building interactions, researchers can optimize the adoption of groundbreaking medical devices. The success of these endeavors is often marked by strategic exits, be it through acquisition, IPO, or alliances, underscoring the importance of robust business development alongside technical expertise. The medical device development landscape is indeed a testament to the intertwined nature of science, engineering, and market dynamics. It is a sector where the successful commercialization of innovations demands not only a deep understanding of the regulatory environment but also an ability to foster strategic partnerships that can propel a medical device from concept to clinical use.
Tailoring Product Messaging to the Target Audience
Effective communication of a medical device's value is paramount for its adoption and utilization. This necessitates a deep understanding of the emotional and practical needs of all users, which includes not only patients but also clinicians, caregivers, and hospital staff.
Crafting a message that resonates with these diverse stakeholders starts with recognizing the initial, often unconscious, emotional reaction users have when encountering the device. This emotional interaction can significantly influence perceptions of a device's quality and performance, as well as the user's mental state during its operation or application.
To foster a positive emotional connection, it's essential to engage in field research and collaborate with Human Factors Engineers. Through facilitated discussions and activities, we can glean insights into users' emotional states and expectations related to medical procedures or conditions.
For instance, understanding the anxiety a patient feels before an MRI can inform a more empathetic approach to device design and communication. Moreover, about 50% of older adults face challenges with medication adherence, often due to complex prescriptions or incorrect dosages.
Digital health solutions offer a promising avenue to improve adherence and patient outcomes, yet they must be designed with user-friendliness in mind, as some older adults find app settings and reminders cumbersome. Despite these hurdles, a significant majority (83%) of older adults are willing to learn new ways to enhance their well-being. In conclusion, medical devices are not just tools for diagnosis or treatment; they are part of a broader ecosystem that includes the environment in which they are used. The design and messaging must therefore account for the setting and context of use, ensuring that the device is distinguishable and functional within its intended space. By adopting a comprehensive approach to device communication that encompasses user-centered and service design principles, we can achieve not only better adoption rates but also improved compliance, usability, and clinical outcomes.
Conclusion
In conclusion, the rapid evolution of medical device research is driven by innovative strategies and digital marketing techniques. Targeted campaigns aligned with controlled studies are crucial for promoting medical innovations, while digital marketing plays a pivotal role in reaching a broader audience. Enhancing customer experience through user-centered design is essential for improving clinical outcomes.
The integration of Artificial Intelligence (AI) revolutionizes healthcare innovation, from predicting patient health deterioration to enhancing device safety and efficacy. Creating detailed buyer personas streamlines development by finding the right partners offering comprehensive services. Optimizing demand generation and lead nurturing maximizes adoption rates for groundbreaking devices.
Tailoring product messaging to diverse stakeholders is paramount for effective communication. Understanding users' emotional reactions and collaborating with Human Factors Engineers helps craft messages that resonate with target audiences. By embracing these strategies, we shape the future of medical device marketing and advance healthcare technology to improve patient outcomes.




