Introduction
The journey of bringing a medical device from concept to market is intricate and demands a comprehensive range of services. Contract Research Organizations (CROs) play a crucial role in providing the technical expertise and regulatory knowledge necessary for the seamless progression of device development. In this article, we will explore the importance of CRO services in medical devices research, highlighting their role in design, engineering, compliance, and market launch.
Additionally, we will examine a case study that showcases the success of integrating user-centered design (UCD) into medical device development. Furthermore, we will delve into the benefits of partnering with a CRO and the regulatory framework for clinical trials in medical devices research. Join us as we dive into the world of CROs and their significant contributions to the healthcare industry.
The Importance of CRO Services in Medical Devices Research
The journey of bringing a medical device from concept to market is intricate and demands a comprehensive range of services. Contract Research Organizations (CROs) are crucial players in this journey, providing the technical expertise and regulatory knowledge essential for the seamless progression of device development.
CROs are well-equipped to handle the complexities of design, engineering, compliance, and market launch, which positions them as valuable partners in the healthcare industry. A standout CRO not only understands the diverse needs of the healthcare ecosystem—patients, providers, payors, and regulatory bodies—but also balances clinical demands with the economic and market factors critical to the success of medical innovations.
Dr. Thomas Fogarty, a leading figure in medical device innovation, encapsulated this sentiment by asserting that "An idea, by itself, has no importance whatsoever; it is the implementation of that idea and its acceptance by others that brings benefit to our patients." Innovation in medical devices is invigorated by the latest technological advancements, with CROss playing a pivotal role in integrating these technologies into the development process. Their services range from concept generation to the deployment of cutting-edge systems, each designed to meet the evolving demands of the sector and improve patient outcomes. The implementation power that CROs bring to the table proves indispensable in translating ideas into accepted medical therapies and technologies that resonate with all stakeholders involved.
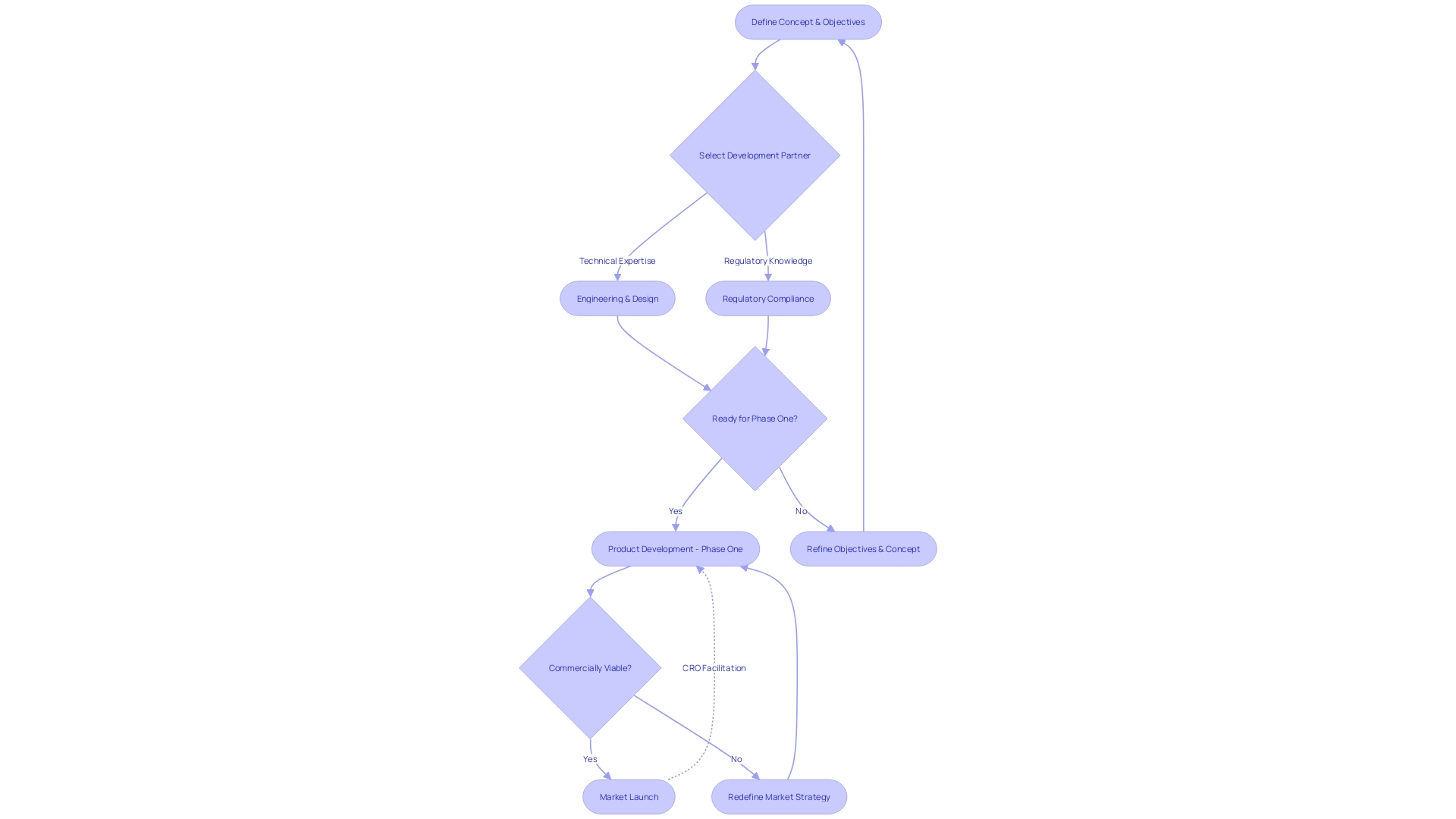
Case Study: Novineon - A Success Story in Medical Device Development
The medical device landscape is intricate and demands a nuanced approach to design and development, particularly when aligning with regulatory standards. Taking cues from Novineon's experience, we witness how integrating user-centered design (UCD) into the creation of medical devices is pivotal.
This approach, which emphasizes a comprehensive understanding of the diverse roles in healthcare—from patients to clinicians and support staff—ensures that medical devices are not only effective but seamlessly integrated into the practical workflows of healthcare settings. Working with a CRO allowed Novineon to refine their device to meet these multifaceted user needs through rigorous usability testing and iterative design processes, culminating in a product that resonates with all stakeholders.
The significance of service design in medical device development is underscored by the collaborative effort to address the expectations and experiences of each individual interacting with the device. With the global medical technology market anticipating substantial growth, reaching estimated revenues of US$215.80 billion by 2024, and covering products that range from imaging instruments to implants, a user-focused methodology is more crucial than ever. Within this expansive market—which includes sectors like In Vitro Diagnostics and Medical Devices—the success hinges on the ability to offer innovative and accessible healthcare solutions. Novineon's journey presents a strong case for the effectiveness and relevance of employing UCD and partnering with a CRO to expertly navigate the regulatory environment and actualize a device that is truly attuned to the user's experience in medical technology development.
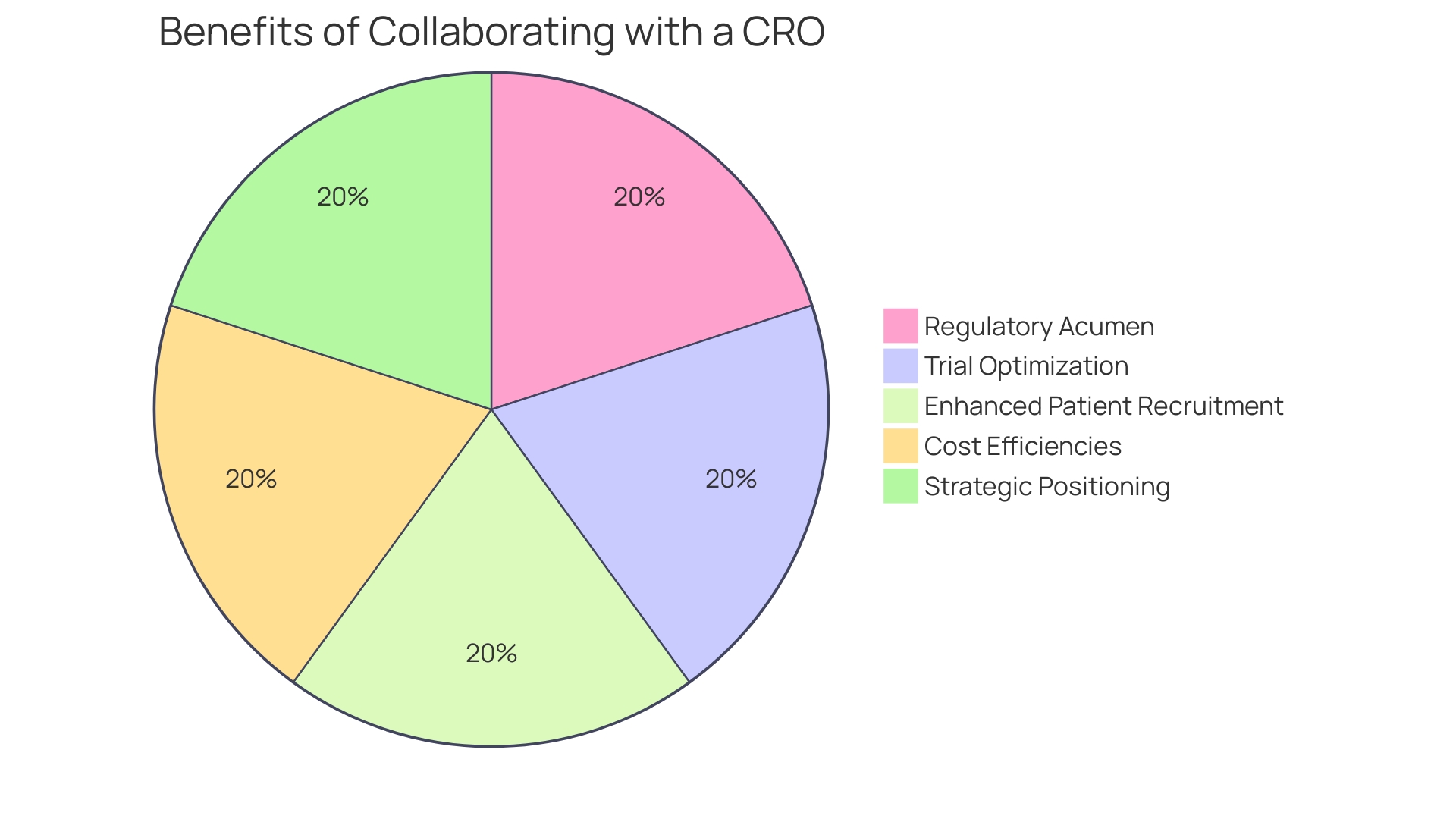
Benefits of Partnering with a CRO for Medical Devices Research
Expanding on the benefits of collaboration with a Contract Research Organization (CRO), it is clear that medical device companies can gain substantially from their regulatory acumen and trial optimization. Drawing from the experience of a business development leader with deep insight into healthcare startups, the embrace of strategic partnerships, such as those with CROss, can be a decisive factor in a successful exit strategy—whether through acquisition, IPO, or licensing deals.
Medical device development, especially in the digital health space, demands not just expertise in hardware and software integration, but a demonstrated capability to usher new products from concept to market. This complex journey is typified by a study design grounded in a clear research question, and meticulous planning to control for confounding variables and biases to ensure credible outcomes.
CROs can deliver enhanced patient recruitment strategies and cost efficiencies, which are critical elements in this process. The alignment of a medical device company with a CRO can serve as testimony to its capacity for successful product launches—a trait highly valued by investors and acquirers alike, as evidenced in the thematic intelligence curated by foremost industry experts. Utilizing leading systems and solutions as identified by industry research, alongside a CRO's proficiency in navigating regulatory landscapes, medical device companies can pivot their strategic position, facilitating not only market entry but also potential lucrative business opportunities.
Regulatory Framework for Clinical Trials in Medical Devices Research
Comprehending the intricate regulatory environment is a cornerstone for R&D in medical device clinical trials. Different FDA classifications exist—Class I, II, and III—reflecting varying levels of risk to patients. The classification informs which registration pathway, be it 510(k), PMA, or the De Novo process, should be chosen for FDA clearance, approval, or grant.
It's crucial to navigate these regulations accurately, as even regulatory professionals find the terminology challenging. A case in point is the differentiation between 'Registered', 'Cleared', 'Approved', and 'Granted' statuses, each signaling a distinctive compliant stage in the device's market authorization process. The FDA emphasizes the importance of 'transparency', a factor critical in enhancing the 'explainability' of the medical logic driving device operation and decisions.
For instance, a device's medical purpose, functional dynamics, and its integration into health care workflows should be lucid to ensure it aligns seamlessly with the user's environment. Describing the intended user, environments, and target populations adds another layer of clarity. Notably, how the device contributes to health care decisions or a health professional's judgment should be crystal clear, ensuring that all potential risks and patient outcomes are comprehensively conveyed and understood.
Conclusion
In conclusion, CROs play a crucial role in the development of medical devices, providing technical expertise and regulatory knowledge for seamless progress. Integrating user-centered design and partnering with CROs refine devices through usability testing, leading to products that resonate with all stakeholders.
Collaborating with CROs offers benefits such as regulatory acumen, trial optimization, and enhanced patient recruitment strategies. Understanding the regulatory framework is vital, ensuring devices align with user environments and contribute to healthcare decisions. Overall, CROs are indispensable for medical device development, contributing to improved outcomes and the success of innovative devices.




