Introduction
Contract Research Organizations (CROs) play a pivotal role in advancing medicine by providing comprehensive services in drug development. They go beyond clinical trial oversight, offering assistance to various stakeholders, including medical device firms, academic bodies, and healthcare institutions.
CROs also facilitate patient access to clinical trials, overcoming logistical challenges and enabling vital treatments. Collaboration is essential in CRO research, bringing together expertise and resources to drive innovation.
Regulatory compliance ensures patient safety, data integrity, and credibility in research outcomes. Successful case studies highlight the impact of CROs on medical progress. Emerging trends, such as the use of artificial intelligence (AI) and digital transformation, are shaping the future of CRO research and development. Through these advancements, CROs are overcoming challenges and driving the industry towards a more efficient and globally accessible future.
Understanding CRO Research and Development
Contract Research Organizations (CROs) are pivotal in advancing the frontiers of medicine for the pharmaceutical and biotechnology sectors by providing extensive services that span the complete spectrum of drug development. Organizations such as CMIC Group have pioneered the CRO model in Japan, continuously innovating to deliver comprehensive solutions. These services reach beyond the scope of traditional clinical trial oversight, encompassing contract development and manufacturing, healthcare solutions, as well as sales and marketing support, particularly for navigating markets like Japan.
CROs are not limited to serving large pharmaceutical entities; they also offer specialized assistance to medical device firms, academic bodies, bio-ventures, and healthcare institutions, tailoring their support to the distinct requirements of each stakeholder. The critical function of CROss is further underscored by their role in facilitating patient access to clinical trials, a process that can be daunting for patients with rare diseases or those in remote locations. The case of a patient with a rare condition in rural Pennsylvania needing to travel internationally for a trial in Turkey illustrates the logistical challenges that CROss help to overcome, thereby enabling access to vital treatments.
Additionally, Bioaccess™, a US-based CRO, exemplifies the expansion of the industry by bridging the gap between innovative medical technology firms and the untapped potential of clinical research in Latin America. In conjunction with research funding support from institutions like the National Institutes of Health and private foundations, CROss can concentrate on the innovation of new drug molecules and the enhancement of their delivery and efficacy. This strategic emphasis on prevalent health issues, such as heart disease and diabetes, showcases the essential role that CROss play in propelling medical progress and improving health outcomes.
The Importance of Collaboration in CRO Research and Development
The landscape of clinical research, particularly within the realm of Type 1 diabetes, is being revolutionized through the power of collaboration. Take for instance the ELSA project, which is currently screening 20,000 children in the UK to identify those at risk of developing Type 1 diabetes.
The project seeks to facilitate early intervention and capitalize on a new medication that has shown promise in delaying the onset of the disease. Such an endeavor is not a solo mission but a symphony of shared expertise and resources, uniting researchers, sponsors, and regulatory bodies in a harmonious effort to innovate and expedite the research process.
The collaborative approach within these projects is multidimensional, involving interdisciplinary teams that blend medicine, engineering, and social sciences, thus ensuring a holistic view of the healthcare challenges being tackled. Indeed, the narrative that innovation is the domain of solitary 'star scientists' is rapidly giving way to the reality that high-impact research is a collective pursuit. As the number of authors on scientific publications and patents has swelled, it's evident that the 'constellation' of collaborators is essential to driving advancements. This shared pursuit not only enhances the relevance and quality of research but also ensures that the solutions developed are robust, addressing user experience and psychological well-being alongside medical efficacy.
Benefits of Regulatory Compliance in CRO Research and Development
Regulatory compliance serves as a crucial bridge between the innovation of clinical research organizations (CROs) and the public's well-being. It is imperative that CROss adhere to guidelines set forth by regulatory bodies like the FDA, EMA, and the emerging EU AI Act, especially when implementing cutting-edge technologies such as AI and ML. This commitment to compliance not only ensures patient safety and data integrity but also fosters trust and credibility in research outcomes.
The rapid evolution of science and technology presents both opportunities and challenges, with regulatory frameworks often lagging behind—termed as the pacing problem. Regulatory authorities have established innovation offices to bridge this gap, creating a synergistic environment where clinical stakeholders and regulators collaborate. This proactive approach is essential, as success in the industry is gauged by regulatory approvals.
Drawing parallels to other industries, such as utilities in California, where a few companies manage significant wildfire risks, the CRO field must also manage compliance proactively. Effective regulation requires influencing corporate behavior to align with societal interests. As new guidelines are proposed to address the compliance concerns associated with AI, CROs must navigate this landscape carefully, ensuring that innovation does not outpace safety and ethical considerations.
Successful Case Studies in CRO Research and Development
The landscape of clinical research organizations (CROs) is ever-evolving, with parallels that can be drawn to other cutting-edge fields such as frontier AI laboratories. Much like the concentrated risk of catastrophic wildfires in California, where three utilities cover most of the state, the CRO field also experiences pivotal moments shaped by a few key players.
The examination of successful case studies in CRO research reveals the profound impact such organizations can have on medical progress. These instances of innovation and breakthroughs underscore the essential elements that foster triumphant research endeavors, providing a blueprint for future success in the realm of medical advancements.
Emerging Trends and Opportunities in CRO Research and Development
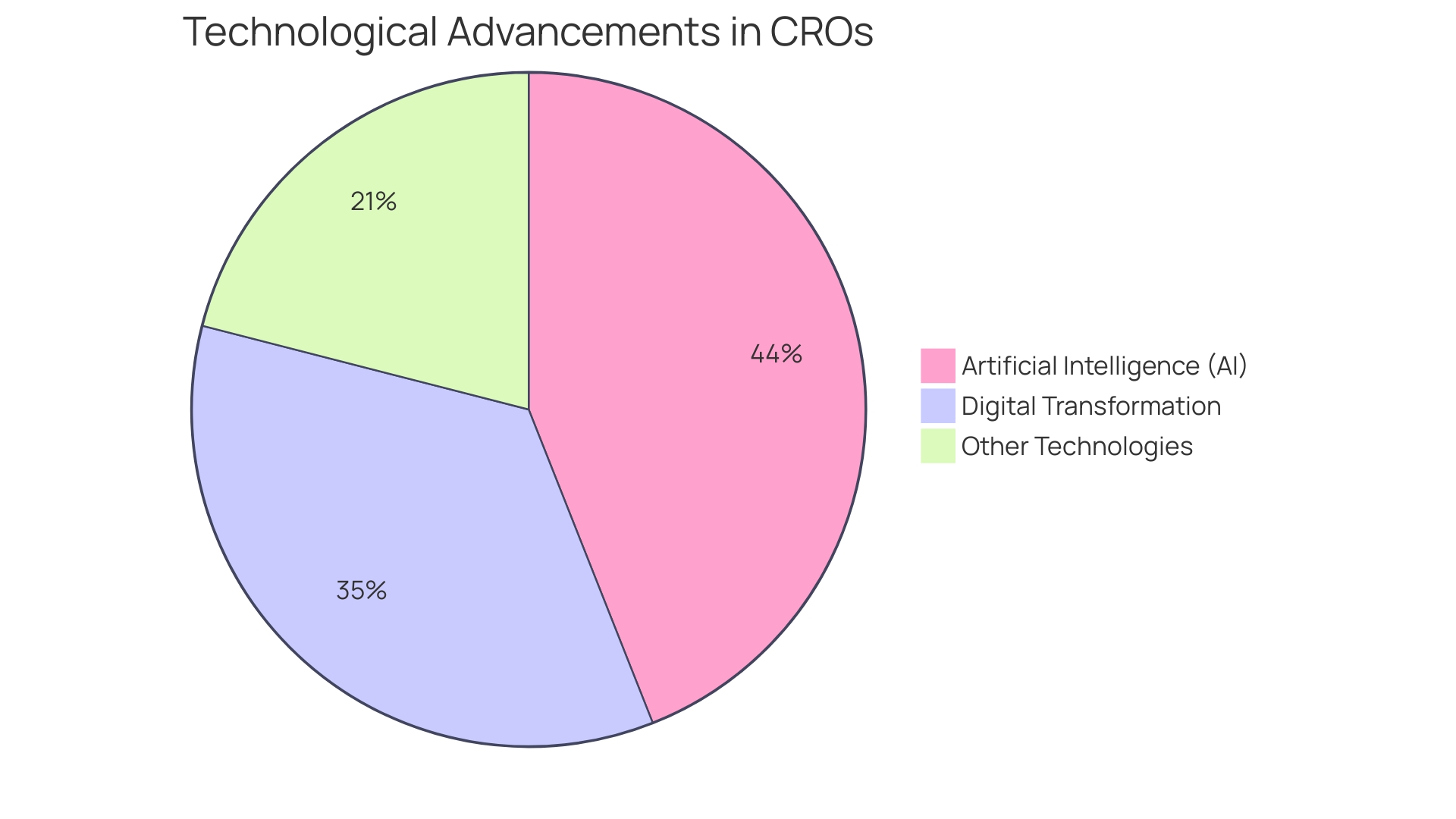
In the dynamic realm of clinical research organizations (CROs), technological advancements and methodological innovations are paving the way for the future. Artificial intelligence (AI) is at the forefront, revolutionizing the industry by enhancing the precision of eligibility criteria for clinical trials.
This is crucial as overly restrictive criteria can lead to insufficient enrollment, while overly broad criteria increase variability and management costs. Ai's capability to estimate the number of eligible patients based on specific criteria is streamlining this process, ensuring trials are both effective and cost-efficient.
Additionally, the digital transformation within regions like the Middle East is propelling the growth of CRO software markets. With the region's shift towards digital solutions and a tech-savvy consumer base, demand for software that optimizes conversion rates on digital platforms is on the rise.
This reflects a broader trend where businesses, recovering from periods of crisis management, are now harnessing market research and AI insights for growth and brand resilience. The integration of AI into research has exponentially increased the speed and scope of data analysis, enabling smarter business decisions. However, this rapid development also highlights a skills gap in market research that businesses must address. By embracing these emerging technologies and methodologies, CRO research and development can continue to advance, overcoming logistical challenges—such as those faced by a patient in rural Pennsylvania needing to travel to Turkey for a trial—and driving the industry toward a more efficient and globally accessible future.
Conclusion
In conclusion, Contract Research Organizations (CROs) are instrumental in advancing medicine through their comprehensive services in drug development. They go beyond clinical trial oversight and provide assistance to various stakeholders, including medical device firms, academic bodies, and healthcare institutions. CROs play a critical role in facilitating patient access to clinical trials, overcoming logistical challenges and enabling vital treatments.
Collaboration is key in CRO research and development, bringing together expertise and resources to drive innovation. Projects like the ELSA project highlight the power of collaboration in revolutionizing clinical research. By uniting researchers, sponsors, and regulatory bodies, CROs can expedite the research process and ensure a holistic view of healthcare challenges.
Regulatory compliance is essential for CROs to ensure patient safety, data integrity, and credibility in research outcomes. Adhering to guidelines set by regulatory bodies fosters trust and credibility in the industry. As CROs embrace cutting-edge technologies like AI and ML, proactive compliance management becomes crucial to align innovation with safety and ethical considerations.
Successful case studies demonstrate the profound impact of CROs on medical progress. These instances of innovation provide a blueprint for future success in medical advancements. Emerging trends such as AI and digital transformation are shaping the future of CRO research and development.
AI enhances precision in eligibility criteria for clinical trials while digital solutions optimize conversion rates on digital platforms. By embracing these emerging technologies and methodologies, CRO research and development can overcome challenges and drive the industry towards a more efficient and globally accessible future. The dynamic landscape of CROs continues to evolve as they contribute significantly to advancing medicine and improving health outcomes worldwide.




