Introduction
Clinical trials are the backbone of medical research, shaping the future of healthcare by evaluating new treatments and interventions. These trials not only determine the safety and effectiveness of novel therapies but also navigate complex ethical, legal, and social considerations. However, there are challenges to overcome, such as patient recruitment and the integration of trials into clinical practice.
In addition, marketing strategies and content creation play a crucial role in attracting diverse participants and ensuring a patient-centric approach. This article delves into the importance of clinical trials, the challenges they face, and the role of marketing and content in advancing medical research. It also includes a case study that highlights the impact of clinical trial companies in pushing the boundaries of medical science and offering hope to patients worldwide.
The Importance of Clinical Trials in Medical Research
Clinical trial companies are at the forefront of medical innovation, undertaking the meticulous process of evaluating new treatments and interventions. Their work is essential in determining the safety and effectiveness of novel therapies, which, if proven successful, can revolutionize patient care.
As highlighted by American Clinical Research Services, these trials are not merely a scientific endeavor but are deeply rooted in ethical, legal, and social considerations. They navigate complex international regulations and strive to align their research with the overarching goal of enhancing the human condition.
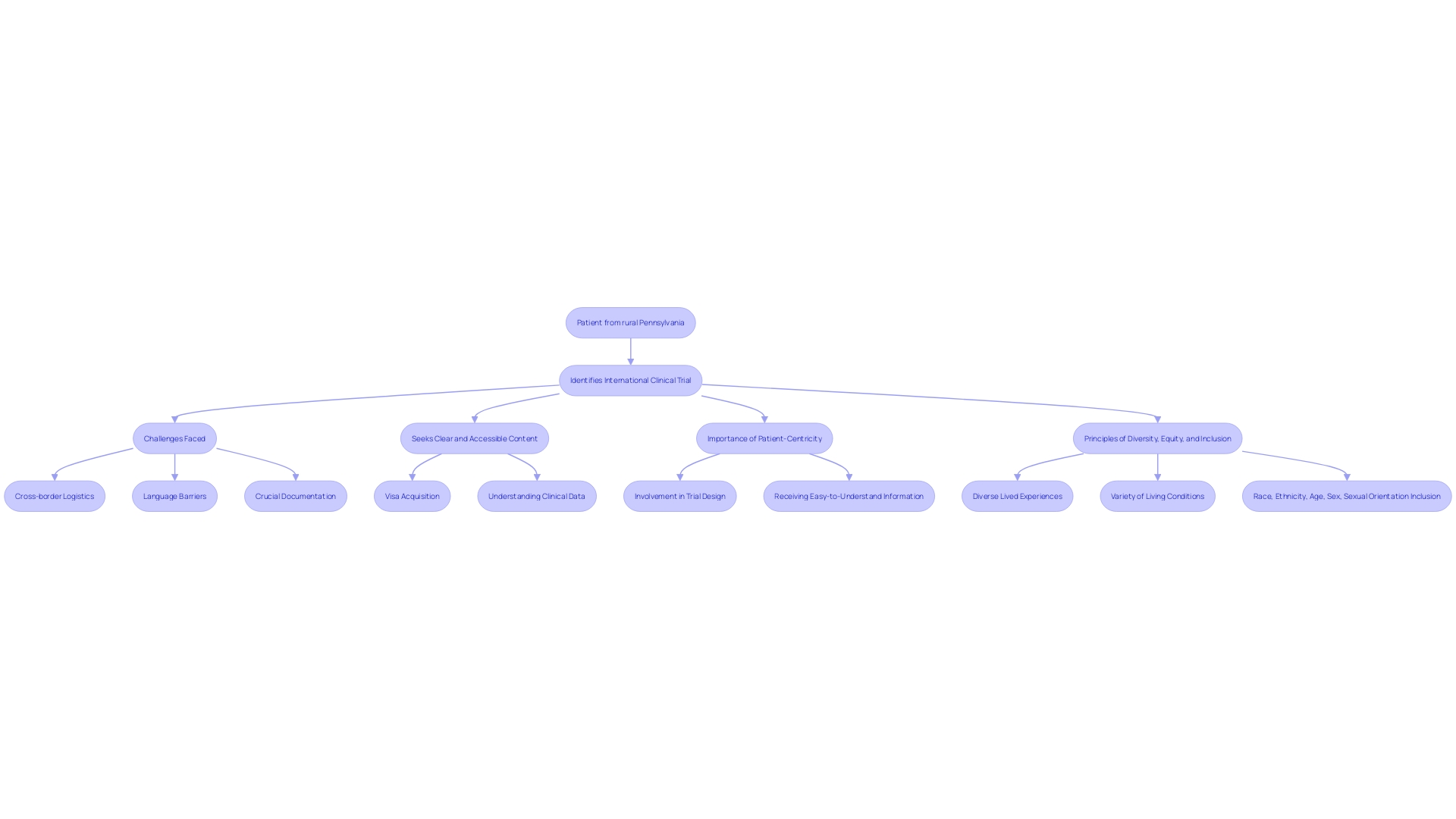
Clinical trials can present logistical challenges, especially for patients with rare diseases seeking treatment abroad. For instance, a patient from rural Pennsylvania may face daunting obstacles when considering a trial in Turkey, from securing visas to overcoming language barriers.
Yet, advancements in technology and online platforms have simplified the process of finding and qualifying for clinical trials. Companies like EmergingMed provide tailored tools that assist patients in locating relevant studies, easing the burden on those in need.
Nonetheless, participation in clinical trials often incurs additional costs for travel and time away from work, which patients must consider. The integration of clinical trials into regular clinical practice is a topic of growing importance, as discussed at the first JAMA Summit. Despite the registration of thousands of randomized controlled trials (RCTs) annually, there is a disconnect between these trials and the realities of clinical practice. As noted by Gregory Curfman, executive editor for JAMA, and Derek Angus of the University of Pittsburgh School of Medicine, this misalignment can lead to inefficiencies and limited impact of trials. It underscores the need for clinical trial companies to design studies that are more reflective of clinical settings, thereby ensuring that the insights gained are both relevant and applicable to patient care.
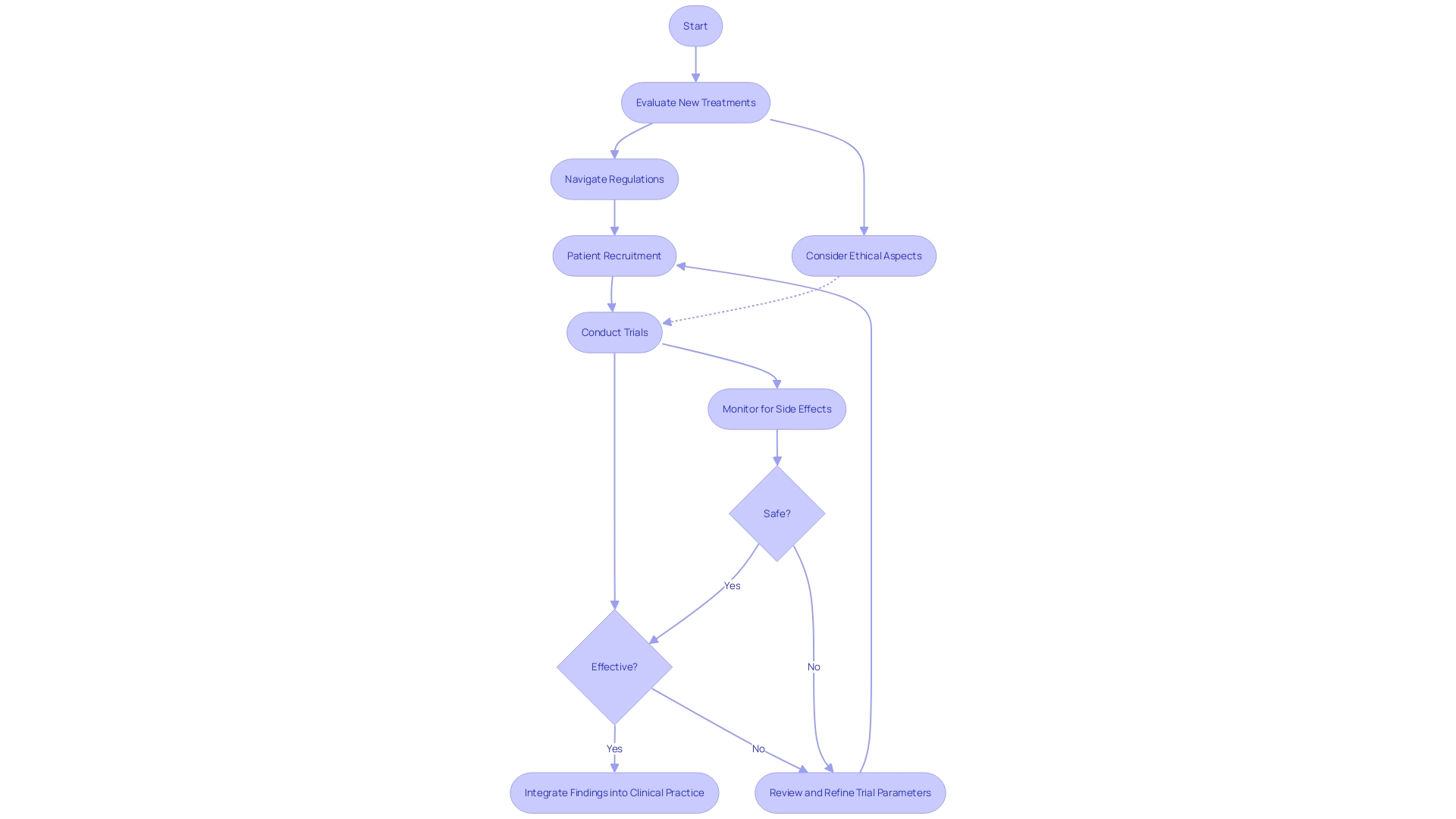
Challenges in Clinical Trial Recruitment
Clinical trial companies are tasked with the crucial role of ensuring that their studies reflect a diverse and robust participant pool, which is foundational to the success and reliability of clinical trials. However, this is often impeded by the multifaceted challenge of patient recruitment. From stringent eligibility criteria to logistical obstacles, these hurdles can significantly impede the enrollment process.
In addressing these barriers, companies must navigate complexities such as geographic limitations, as evidenced by a patient in rural Pennsylvania who faces an arduous journey to participate in a trial in Turkey. The burden of organizing international travel and overcoming language barriers is a stark example of the recruitment difficulties faced by both patients and companies. Emphasizing patient centricity, as highlighted by Daniel J Herron of RWS, entails prioritizing the patient experience in the trial design, ensuring that information is comprehensible, and involving patients in the planning process.
This approach not only facilitates better recruitment but also encourages a more inclusive environment. It recognizes the necessity of diversity, equity, and inclusion (DEI) in clinical research, as individuals from different backgrounds and with diverse lived experiences may respond distinctively to the same disease. Hence, the recruitment strategies of clinical trial companies must be adaptive and considerate of the multifaceted needs of potential participants, thereby enabling a more equitable access to clinical trials across various communities.
Marketing Strategies for Clinical Trials
In the dynamic landscape of clinical trial marketing, the digital transformation and integration of AI have become pivotal. Today's pharmaceutical consumers are accustomed to the exceptional levels of service and information offered by tech giants like Amazon, Netflix, and Meta, setting a high bar for customer experiences across industries.
This shift necessitates a customer-centric approach in clinical trial marketing strategies. Utilizing advanced technologies such as Machine Learning and Propensity Modeling, clinical trial companies can enhance their marketing efforts to mirror the personalization and convenience that consumers now expect.
For instance, consider the scenario of a patient in rural Pennsylvania with an ultra-rare disease, who is presented with the chance to join a clinical trial in Turkey. The marketing message for this trial must not only inform but also support the potential participant through complexities such as visa applications and travel logistics, using clear and accessible language. Ken Getz, an industry expert, underscores the importance of harmonizing excellent science with efficient execution, highlighting the role of newer technologies in improving operational activities. By addressing the unique challenges faced by patients and employing a nuanced understanding of both consumer and medical marketing, clinical trial companies can effectively communicate the benefits of participation, thereby attracting a diverse participant pool and driving forward medical advancements.
The Role of Content Marketing in Clinical Trials
To effectively engage and educate prospective participants, clinical trial companies must craft content that not only informs but also addresses the challenges and concerns faced by patients. A case in point is the patient from rural Pennsylvania, grappling with an ultra-rare disease and the complexities of participating in an international clinical trial. This patient's journey underscores the need for clear, accessible content that aids in navigating cross-border logistics, language barriers, and crucial documentation.
By producing comprehensive educational materials that cover everything from visa acquisition to understanding new clinical data, companies can significantly improve the patient experience. As noted by Daniel J Herron of RWS, emphasizing patient-centricity is key, which includes involving patients in trial design and ensuring information is understandable, reflecting the principles of diversity, equity, and inclusion. This approach not only builds trust but also ensures a broad range of patient experiences are represented, ultimately enhancing the relevance and impact of clinical trials.
Case Study: Advancing Medical Research with American Clinical Research Services
American Clinical Research Services (ACRS) is a leading clinical trial company dedicated to advancing medical research. In a recent case study, ACRS conducted a phase III clinical trial to evaluate the efficacy and safety of a novel cancer treatment. The study involved a diverse group of patients from different demographic backgrounds.
Results and Impact of the Case Study
A recent phase III clinical trial spearheaded by ACRS has brought new hope to the oncology community. The cutting-edge cancer treatment under investigation not only shrank tumors significantly but also extended the lives of patients, marking a substantial stride in cancer care.
These groundbreaking results were recognized by a leading medical journal, underscoring the critical contribution of clinical trial companies in pushing the boundaries of medical science. The trial's success is a testament to the pivotal role these companies play, not just in research and development, but in offering hope to patients even in the most remote locations.
For instance, consider the challenges faced by a patient with a rare disease in rural Pennsylvania, who must navigate the complexities of international travel to participate in a life-saving trial in Turkey. From visa procurement to language barriers and coordinating travel, the logistical hurdles are immense. This scenario underscores the need for clinical trial companies to facilitate not only the development of new treatments but also to ensure patient accessibility and support at every step of the process.
Conclusion
Clinical trial companies play a vital role in advancing medical research and revolutionizing patient care. These trials evaluate novel therapies, considering ethical and social aspects. Challenges like patient recruitment and integration into clinical practice must be addressed.
Patient recruitment is a complex challenge that requires adaptive strategies for diverse participation. Emphasizing patient centricity promotes diversity, equity, and inclusion in research. Marketing strategies and content creation are crucial for attracting participants.
Using a customer-centric approach and advanced technologies, clinical trial companies can effectively communicate the benefits of participation. Content marketing plays a key role in engaging and educating prospective participants. Clear content addressing patient challenges improves the experience and ensures diverse representation.
The case study by American Clinical Research Services (ACRS) showcases the impact of clinical trial companies. Their phase III trial evaluating a cancer treatment brought hope to patients by shrinking tumors and extending lives. In summary, clinical trials are essential for medical research but face challenges in recruitment and integration.
Marketing strategies and content creation are vital for attracting diverse participants. The ACRS case study demonstrates the significant impact of clinical trial companies in pushing boundaries and offering hope to patients globally. Through their dedication, we can enhance patient care worldwide.
Join us today and be part of the future of medical research and patient care!




