Introduction
Cardiovascular diseases are a major global health concern, responsible for a significant number of deaths each year. In order to address this critical issue, cardiovascular research plays a crucial role in developing effective treatments and interventions.
However, this type of research presents unique challenges, including the need for diverse and representative patient populations and long-term follow-up. Partnerships with cardiovascular Contract Research Organizations (CROs) have emerged as a key solution in advancing medical research in the field of cardiology.
These CROs provide specialized expertise in trial design, patient recruitment, and data integrity, streamlining the complex process of clinical trials. Through successful collaborations with CROs, research institutes have been able to overcome barriers and achieve important milestones in evaluating novel treatments for cardiovascular diseases. This article explores the importance of cardiovascular research, the challenges it faces, the role of CROs, and the benefits of partnering with them. By delving into these topics, readers will gain a comprehensive understanding of the significance of cardiovascular research and its potential for improving global cardiac health.
The Importance of Cardiovascular Research
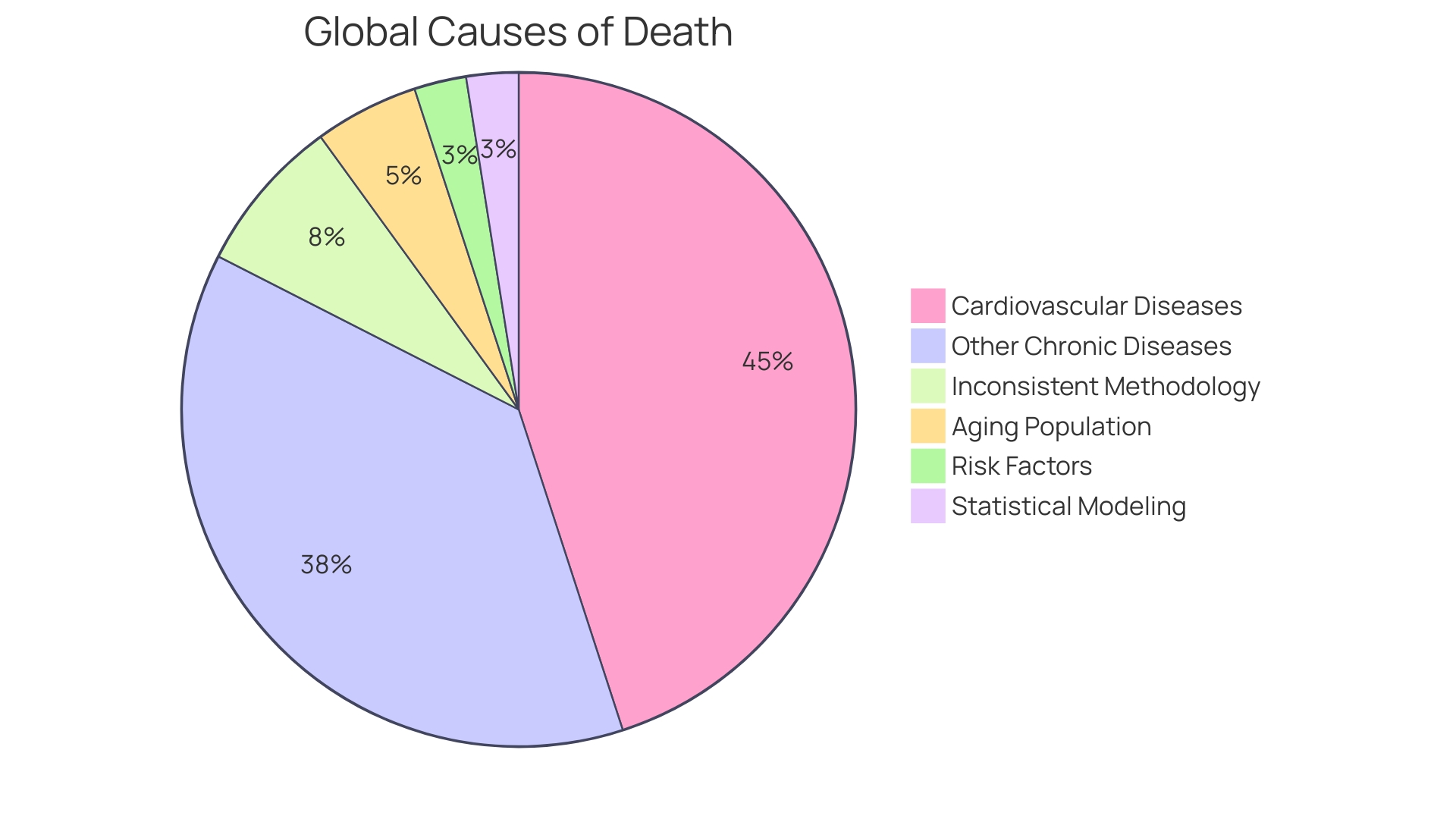
Cardiovascular diseases (CVDs) stand as a substantial public health challenge due to their status as the most common cause of death worldwide. In 2019, approximately 18 million deaths were attributed to CVDs, representing a third of all global fatalities.
This alarming statistic underscores the critical nature of cardiovascular research. Clinical trials within this domain are paramount—they are the conduit through which new treatments for heart disease, hypertension, stroke, and other vascular conditions are evaluated for safety and effectiveness.
The complexity of CVDs demands a multi-faceted research approach. Factors such as genetics, behavior, socioeconomic and environmental conditions create a labyrinth of interdependent variables influencing cardiovascular health.
Policymakers, healthcare providers, and researchers grapple with identifying the most impactful interventions in this intricate landscape, where single-factor studies are plentiful, but comprehensive tools for intervention prioritization remain scarce. Though the global death toll from CVDs rose from 14 million in 2000 to nearly 18 million in 2019, driven by population growth and aging, particularly in Asia, alongside escalating risk factors, there is a silver lining. Many countries have witnessed declining death rates from cardiovascular diseases thanks to progressive interventions and research breakthroughs. With limited resources for interventions, an understanding of cardiovascular systems as a whole—including key leverage points—is essential for health optimization. Cardiovascular clinical trials are key elements in this complex system, offering insights that can lead to decisive actions in the battle against the world's leading cause of mortality.
Challenges in Cardiovascular Clinical Trials
While cardiovascular clinical trials are crucial for medical advancements, they also present several challenges. One of the main challenges is recruiting a diverse and representative patient population.
Cardiovascular conditions can vary among different demographics, and it's important to ensure that participants in clinical trials reflect the real-world patient population. Another challenge is the long-term follow-up required in cardiovascular research. Many cardiovascular diseases require continuous monitoring and assessment, which can be resource-intensive and time-consuming for both the researchers and the participants.
The Role of Cardiovascular CROs in Advancing Medical Research
Cardiovascular Contract Research Organizations (CROss) are at the forefront of driving innovations in the realm of heart health. The specific focus of these CROss on cardiology allows them to offer nuanced expertise in trial design tailored to cardiac ailments, navigating the intricacies of patient recruitment, and ensuring impeccable data integrity.
Streamlining compliance with myriad regulatory standards is another forte of such organizations. A heartening case comes from a scenario where a Pennsylvania resident grapples with an ultra-rare disease and finds hope many miles away in a clinical trial in Turkey - a situation that not only embodies the complexity of accessing treatment but also the logistical labyrinth encountered when traversing borders for the promise of health. These challenges underscore the importance of cardiovascular CROss in demystifying the clinical trial journey for participants, thus potentially ushering in an era of more accessible and egalitarian healthcare as underscored in the philosophy behind the advancing AI in medical research, striving for transparency and explanations that patients can easily comprehend.
Case Study: Successful Collaboration with a Cardiovascular CRO
A cardiovascular research institute embarking on an ambitious clinical trial to assess a novel heart failure treatment encountered significant barriers. One crucial issue was the ineffective patient recruitment, a common hurdle given that sufficient participant numbers are vital for trials' statistical robustness.
To ameliorate this, the institute formed a strategic partnership with a cardiovascular Clinical Research Organization (CRO) renowned for its proficiency in navigating the intricacies of cardiovascular trials. Leveraging the CRO's adept recruitment tactics, the trial identified and enrolled suitable patients more efficiently.
Furthermore, the CRO augmented the trial's integrity by offering meticulous data management services, ensuring accurate and reliable results. This symbiotic relationship culminated in the trial meeting its completion deadline, thereby cementing the CRO's role as a linchpin in the successful evaluation of the heart failure intervention. Such alliances are indispensable in the realm of clinical trials, where the advent and validation of innovative treatments hinge on the meticulous execution of multi-phase studies. With phase two trials not only assessing safety but also the efficacy of treatments among affected individuals, the strategic use of specialized CROs can be the difference between trial success and stagnation.
Benefits of Partnering with a Cardiovascular CRO
Collaborating with a cardiovascular Contract Research Organization (CRO) is instrumental for research entities and biopharmaceutical firms, particularly in addressing the multifaceted healthcare ecosystem's needs. A CRO brings forth a cadre of specialists well-versed in the complexities of cardiovascular studies.
Their involvement promotes an enhanced trial design, a more efficient participant recruitment pathway, and superior data handling methodologies. Additionally, they are pivotal in curtailing both the duration and expenditures associated with clinical trials, streamlining operations through their established resources and sophisticated infrastructure, which in turn expedites the research continuum.
A poignant example can be drawn from a patient with a rare disease in rural Pennsylvania pondering participation in a critical trial overseas, encapsulating the intricate logistics and regulatory intricacies they face from visa procurement to navigating foreign documentation. It underscores the integral role of CROs in ensuring rigorous regulatory compliance, adherence to industry norms, and validating trial findings—a cornerstone in gaining approval and acceptance within the intricate web of patients, families, healthcare providers, and regulatory bodies. Such strategic partnerships inherently align with the philosophy expressed by Dr. Thomas Fogarty, emphasizing that innovation stretches beyond mere ideation—it necessitates a thorough understanding of the healthcare community's diverse needs, translating ideas into tangible benefits for patients through carefully orchestrated implementation and widespread endorsement.
Conclusion
In conclusion, cardiovascular research plays a critical role in addressing the global health challenge of cardiovascular diseases (CVDs). Clinical trials are essential for evaluating new treatments and interventions for heart disease, hypertension, stroke, and other vascular conditions.
While these trials face challenges, partnerships with cardiovascular Contract Research Organizations (CROs) offer solutions. CVDs are the leading cause of death worldwide, and a multi-faceted research approach is needed due to the complexity of these diseases.
Clinical trials provide valuable insights into the effectiveness and safety of interventions, contributing to declining death rates in many countries. Recruiting diverse patient populations and conducting long-term follow-up are challenges in cardiovascular research.
CROs specializing in cardiovascular studies bring expertise in trial design, patient recruitment, and data integrity. They streamline the complex process, ensuring successful evaluation of treatments.
Collaboration with CROs is crucial for the execution of clinical trials. Strategic partnerships improve patient recruitment and data management, facilitating the timely completion of trials.
Such alliances are indispensable for advancing innovative treatments and achieving trial success. Partnering with cardiovascular CROs offers various benefits.
It enhances trial design, improves participant recruitment, and ensures superior data handling. Additionally, these collaborations reduce the duration and costs associated with trials, streamlining research operations. These partnerships also address regulatory complexities and logistical challenges faced by patients, ensuring compliance and validating trial findings. By aligning with the healthcare community's needs, collaboration with CROs translates scientific ideas into tangible benefits for patients, advancing global cardiac health. In summary, cardiovascular research is crucial for combating CVDs and improving global cardiac health. Collaboration with CROs enables the successful execution of clinical trials, overcoming challenges and driving progress in the field. By investing in cardiovascular research and fostering partnerships, we can work towards a future with improved cardiovascular health outcomes worldwide.




