Introduction
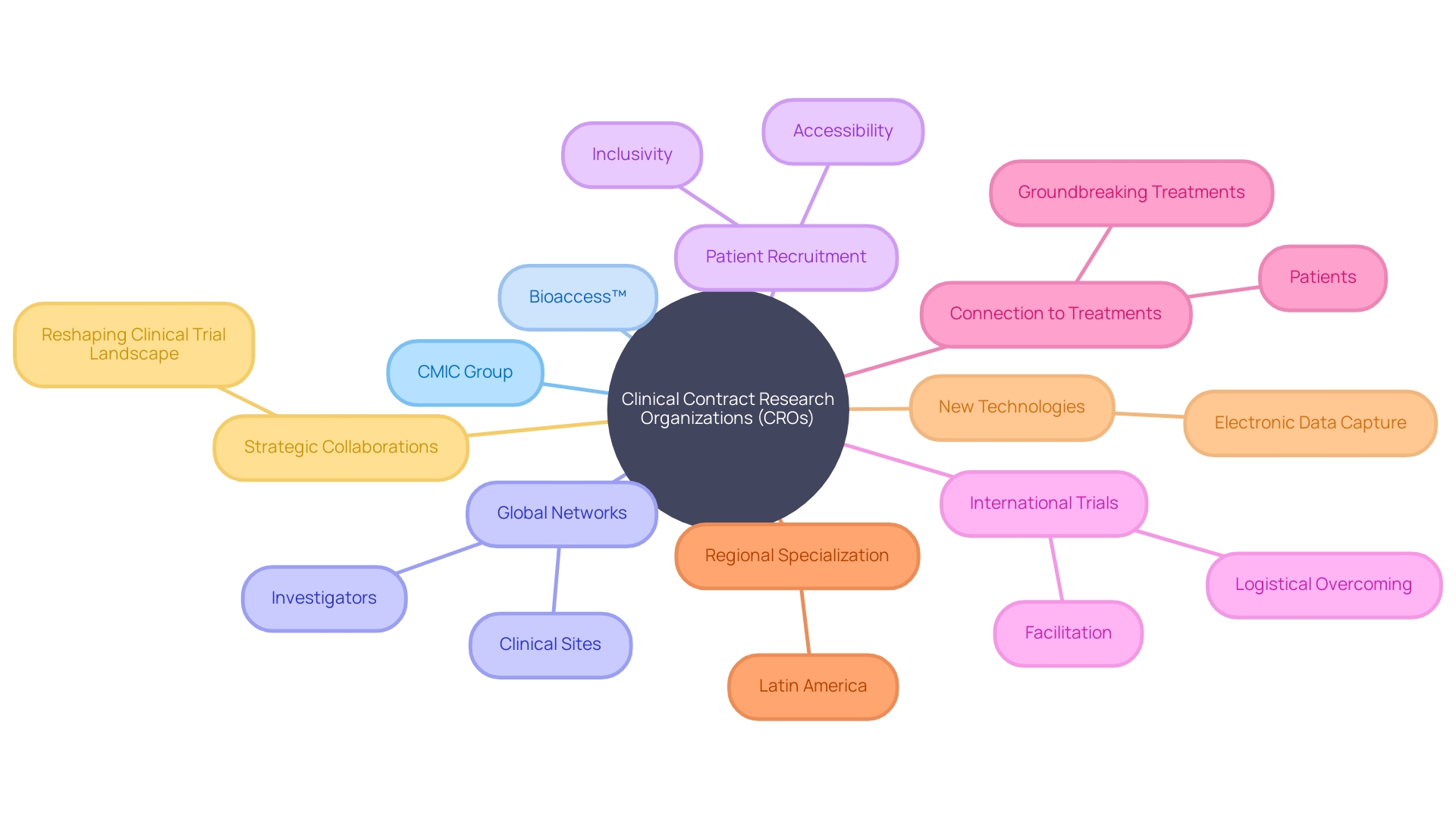
Clinical Contract Research Organizations (CROs) play a crucial role in the realm of pharmaceutical and biotechnology industries, going beyond the mere execution of clinical trials. They tackle a range of complexities across ethical, legal, and social dimensions, navigating issues such as intellectual property, patient compensation, and international logistics.
With the rise of digital therapeutics, CROs must also adapt to the challenges posed by innovative treatments. In this article, we explore the multifaceted responsibilities of CROs, the services they offer, the benefits of partnering with them, and the future of medical research in their hands.
The Role of Clinical Contract Research Organizations (CROs)
The role of Clinical Contract Research Organizations (CROs) extends far beyond merely facilitating the execution of clinical trials. These specialized companies address a myriad of complexities across ethical, legal, and social dimensions, as they support both the pharmaceutical and biotechnology industries. Each clinical study they manage can introduce unique ethical conundrums, requiring a careful consideration of a broad spectrum of issues – from intellectual property to the equity of participant compensation.
A patient offered a trial for an ultra-rare disease internationally, for instance, must navigate not only health concerns but also the intricacies of travel and legal documentation, often without language support. The evolving ethos underscores the necessity for fair treatment of clinical trial participants, acknowledging their contribution towards the greater public health while compensating them for their expenses, efforts, and potential risks, akin to public service roles such as first-responders. Furthermore, the advent of digital therapeutics introduces fresh challenges and underscores the need for adaptable clinical trials.
These innovative treatments, based on software to manage medical disorders and involving the use of devices like smartphones and sensors, require meticulous testing and present insurance coverage hurdles due to their novelty. Addressing these issues requires CROss to be agile and responsive to the shifting landscape, embodying the dual roles of strategic partner and ethical steward. It is clear that the governance landscape of clinical trials necessitates a global and holistic perspective, considering not only the immediate logistics but also the overarching social aim of improving the human condition through scientific advancement.
Services Offered by Clinical Contract Research Organizations
Contract Research Organizations (CROs), exemplified by Bioaccess™, are pivotal in the evolution of pharmaceutical and medical research, offering comprehensive services that facilitate the transition from laboratory research to practical clinical applications. Bioaccess™ specializes in extending the reach of clinical research by tapping into the underexplored regions of Latin America, which presents a valuable opportunity for medtech companies seeking new ground for conducting their studies.
Integral to the services provided by CROss, including the management of detailed study designs and protocols, they adeptly maneuver through the intricate pathways of clinical trials. Their expertise encompasses site selection and extensive feasibility studies, promoting accurate patient recruitment and enrollment strategies.
The approach adopted by such organizations, exemplified by the CMIC Group in Japan, is geared towards overcoming the challenges of drug development. This includes facilitating the participation of patients who may face obstacles such as language barriers and long-distance travel.
Utilizing advanced technology for precise data collection and management, thorough statistical analysis, and maintaining the high standards required for regulatory compliance are also cornerstones of the CRO's role. The benefits for patients, even in remote areas grappling with diseases lacking effective treatments, are significant. Ken Getz's analysis of clinical trial design resonates with the industry's focus on harmonizing robust scientific foundations with enhanced operational efficiency, propelled by electronic data capture and other cutting-edge technologies. The extensive capabilities of CROss span contract development, healthcare solutions, and market entry strategies, highlighting their crucial position in strengthening the pharmaceutical value chain and addressing client needs throughout every phase of drug development.
Benefits of Partnering with Clinical Contract Research Organizations
Clinical Contract Research Organizations (CROs) are pivotal in advancing clinical trial management, and an exemplar in this space is the renowned CMIC Group from Japan, with its impressive track record extending beyond three decades of service. Expert in handling the comprehensive spectrum of the pharmaceutical industry, these organizations leverage a broad network of investigators and clinical sites.
This extensive network fortifies the dynamics of patient recruitment, making trials more inclusive and accessible to varied populations and remote locations. For instance, a patient hailing from rural Pennsylvania with a unique health condition now has the means to participate in an international trial, with the logistical and linguistic obstacles skillfully managed by these adept CRO partners.
Their facilitation extends to navigating complex regulations and travel coordination, effectively connecting patients with groundbreaking treatments. Another example of innovation in this sector is Bioaccess™, a United States-based CRO specializing in the Latin American region.
It acts as a conduit for cutting-edge medtech companies to tap into the region's promising capacity for clinical research, thereby broadening the scope of patient participation in trials. In parallel with these developments, Ken Getz's industry analysis observes a movement towards balancing high-caliber scientific inquiry with astute operational oversight. Embracing new technologies such as electronic data capture, CROs like CMIC Group and Bioaccess™ are revolutionizing the field. They enhance efficiency, fortify compliance with regulatory standards, and conserve valuable resources. Through strategic collaborations, CROss are restructuring the clinical trial landscape, expediting the discovery of novel medical solutions and raising benchmarks in the execution of clinical studies.
The Future of Medical Research with Clinical Contract Research Organizations
The landscape of medical research is on the cusp of a transformative era, buoyed by the strides made by Clinical Contract Research Organizations (CROs). Over three decades after CMIC Group catalyzed the establishment of the CRO sector in Japan, these organizations stand out as central facilitators in the continuum of drug development.
Their role has magnified beyond conventional clinical trial management to incorporate comprehensive services across the pharmaceutical value chain, including development, manufacturing, and even navigating market entry nuances. At the heart of CROs' evolution is an unwavering commitment to patient-centric research and uncompromising scientific integrity.
These entities are not just conducting trials; they are reshaping them to be more inclusive and accessible. For instance, when a patient in rural Pennsylvania with a rare, untreatable illness is given the chance to partake in a groundbreaking clinical study abroad in Turkey, CROss can streamline the daunting process.
From visa procurement to language barriers and travel arrangements - these organizations are instrumental in knitting together disparate aspects that can otherwise hinder participation in pioneering research. CROs are accelerating the transition from laboratory insights to real-world therapeutic applications, knitting together scientific exploration with enhanced healthcare delivery. Their adroitness in adopting novel technologies and methodologies stands to expedite trial timelines and bolster the precision of the findings. As we forge ahead, CROss are certain to be the driving force behind the future discoveries that will redefine the medical landscape.
Conclusion
Clinical Contract Research Organizations (CROs) play a vital role in the pharmaceutical and biotechnology industries, going beyond the mere execution of clinical trials. They navigate complex ethical, legal, and social dimensions, addressing issues such as intellectual property, patient compensation, and international logistics.
The evolving landscape of medical research, particularly with the rise of digital therapeutics, requires CROs to be agile and adaptable to new challenges. CROs offer comprehensive services that facilitate the transition from laboratory research to practical clinical applications.
They excel in managing detailed study designs, site selection, patient recruitment, and regulatory compliance. The expertise of CROs like Bioaccess™ and CMIC Group extends to overcoming language barriers and facilitating participation in trials for remote patients.
Their incorporation of advanced technology ensures precise data collection and analysis, elevating the standards of drug development and benefitting patients worldwide. Partnering with CROs brings numerous benefits, including an extensive network of investigators and clinical sites, enabling more inclusive and accessible trials.
CROs like CMIC Group and Bioaccess™ further expand the reach of clinical research by tapping into underexplored regions. Through strategic collaborations, CROs revolutionize the clinical trial landscape, expediting the discovery of novel medical solutions and setting higher benchmarks for trial execution. The future of medical research lies in the hands of CROs. They are reshaping clinical trials to be more patient-centric, inclusive, and accessible. By adopting novel technologies and methodologies, CROs accelerate the transition from laboratory insights to real-world therapeutic applications. With their unwavering commitment to scientific integrity and patient-centric research, CROs will continue to drive future discoveries that redefine the medical landscape.
Join forces with bioaccess™ today and unlock the full potential of your clinical trials!




