Introduction
Clinical data management plays a crucial role in medical research, ensuring the integrity and reliability of data collected during clinical trials. It involves comprehensive processes, from data collection to analysis, to meet regulatory standards and prioritize participant safety. The success of medical device companies and the approval of devices by regulatory bodies heavily rely on the effective management of clinical trial data.
The integration of real-world data and the push for standardization and interoperability further enhance the validity and inclusivity of study findings. As the healthcare industry embraces digital health records and AI-driven methodologies, the need for standardized tools and best practices in clinical data management becomes paramount. This article explores the importance, objectives, roles, and responsibilities, as well as the tools and technologies used in clinical data management.
It also highlights the benefits and best practices of effective data management and discusses future trends, particularly the integration of AI and ML, in shaping the landscape of clinical data management and patient care.
Importance of Clinical Data Management in Medical Research
The accuracy of medical information is crucial to the accomplishment of medical research. Comprehensive handling of patient information covers the complete process of information from its gathering during trials to the final evaluation. The careful collection and handling of this information is not just about adhering to regulations, but it also protects the welfare and rights of participants, as required by Article 62 of the European Union Medical Device Regulation (EU MDR). The rule highlights that scientific studies must give utmost importance to the rights, safety, dignity, and well-being of subjects above everything else, guaranteeing that the information generated is strong and scientifically valid.
In the medical device sector, especially when developing high-risk devices, the handling of trial information is crucial for ensuring market entry. Information gathered from trials forms the evidentiary basis upon which the safety and efficacy of a medical device are assessed by regulatory bodies. In the United States, information from trials supports around 10-15% of successful 510(k) submissions for Class II devices, while all Class III devices necessitate thorough trials to prove their safety and effectiveness.
The 2024 State of the MedTech Industry Benchmark Report emphasizes the vital importance of trials for medical device companies, with a significant focus on both pre-market and post-market activities. Nevertheless, it is not merely the trials themselves that hold significance; the approaches for information handling are of equal significance. Efficient handling of information in a medical setting must surpass mere collection and storage; it encompasses a thorough procedure that guarantees the production of top-notch, dependable outcomes in compliance with proper medical conduct and local regulations. Without such practices in place, information may fail to accurately reflect study outcomes, potentially rendering it unusable for regulatory approval.
The World Health Organization (WHO) estimates a wide range of medical devices, around two million types, are available in the market globally, which highlights the extent of influence that the handling of healthcare information can have on the well-being of individuals. It is crucial for MedTech companies to navigate the intricacies of information management, avoiding typical traps and utilizing the appropriate tools and resources to uphold the utmost standards of integrity. Real-world information, which includes patient records from everyday healthcare encounters, is increasingly being utilized to supplement traditional clinical trial data, providing a more comprehensive representation of patient populations and improving the reliability of study findings.
In this age of increasing adoption of electronic medical records, the movement towards uniformity and compatibility of health information has gained momentum. This is evident in regions like the United States, where federal initiatives such as the Health Information Technology for Economic and Clinical have spurred progress towards better health information connectivity. As the field of medical information control progresses, its contribution to the progress of medical investigation and the provision of secure, efficient medical devices persists as indispensable as always.
Key Objectives of Clinical Data Management
Thorough management of important information is crucial to the integrity and success of any research study. By utilizing standardized information gathering procedures, clinical researchers can guarantee the precision, reliability, and comprehensiveness of the information gathered. Furthermore, ensuring strong security and privacy measures is crucial for safeguarding patient information and complying with regulations. To accomplish this, healthcare organizations must adopt IT solutions that enable efficient configuration and management of information, taking into account the intricacies of personal information handling.
Integration and interoperability of information across various research studies and databases are fundamental objectives in today's data-driven healthcare environment. By using connected devices, wearables, and decentralized trial solutions, researchers can gather a wealth of insights. Advanced AI-driven methodologies further enhance the capability to extract meaningful insights from diverse sources such as lab results, patient-reported outcomes, and imaging. Establishing standards and guidelines is vital for managing the influx of both traditional and digital sources, ensuring that the information collected can be seamlessly integrated and utilized for informed decision-making in drug development.
The healthcare sector's dedication to security has become more emphasized, with life sciences, biotechnology, and pharmaceutical companies acknowledging the benefits of prioritizing strong security measures. The utilization of virtual data rooms (VDRs) showcases the industry's endeavors to secure sensitive information. These secure cloud-based repositories feature advanced security measures, including encryption, multi-factor authentication, access controls, and audit trails, to safeguard against unauthorized access and misuse of information.
In addition, the utilization of Clinical Data Repositories (CDRs) presents a real-time, patient-centered approach to information management. CDRs facilitate immediate access to comprehensive clinical information, enabling healthcare providers to make informed treatment decisions. By providing a longitudinal view of a patient's medical history, CDRs can reduce redundancies and streamline care. Utilizing CDRs for predictive modeling and risk assessment is also becoming more and more prevalent, thanks to the ease of information extraction for intelligent algorithmic analysis.
To summarize, the success of efficient handling of medical information depends on a strategic method that integrates up-to-date information technology solutions, strict security procedures, and a dedication to information standards. By doing this, healthcare institutions can guarantee the quality and privacy of medical information while promoting advancement in medical research.
Roles and Responsibilities in Clinical Data Management
The discipline of clinical information management is multifaceted, encompassing various responsibilities essential to the integrity of clinical research. Managers play a crucial role in coordinating the lifecycle of information—from careful collection to thorough cleansing, rigorous validation, and insightful analysis. By forming cooperative alliances with biostatisticians, study coordinators, and IT experts, they advocate for the veracity and quality of information.
Clinical Data Repositories (CDRs) represent the progress of information administration. These databases that prioritize patients are not only continuously updated but also organized to facilitate the efficient retrieval of medical information. They serve as a bedrock for medical decision-making, offering a longitudinal view of a patient's medical history, prior procedures, and test results, thereby curtailing redundant testing and streamlining care delivery. Furthermore, CDRs are priceless in facilitating risk anticipation and modeling via intelligent algorithms that utilize the easily accessible medical information.
The significance of stellar control over medical information cannot be exaggerated, especially considering that the well-being of human participants in trials depends on it. This information constitutes the essence of regulatory submissions, validating the safety and effectiveness of medical devices intended for market release. With the WHO reporting an astounding two million varieties of medical devices in circulation worldwide, the role of information control in safeguarding patient safety and drawing reliable conclusions from trials becomes even more crucial.
Recent updates in the European good practice guidelines, particularly the section on data governance, underscore the necessity for robust data management practices. As per Silvia Perez, director of quality compliance at AstraZeneca, this clarity is pivotal in ensuring participant safety and the reliability of trial outcomes. It also calls for a meticulous evaluation of the scientific objectives of clinical trials in relation to the risks they pose, with a focus on activities crucial to the trial's goals.
Within the healthcare industry, the process of handling information faces various obstacles, which include protecting the privacy of individuals and complying with strict rules. The integration of business acumen, scientific insight, and technological innovation is imperative to navigate these complexities. As the industry struggles with the quality of patient information, questions arise about the dependability of the information gathered and its influence on healthcare objectives, prompting the necessity for ongoing enhancement in information management strategies.
Tools and Technologies Used in Clinical Data Management
CDM is an essential stage in medical research, which results in the production of high-quality, reliable, and statistically sound information from trials. With the growing intricacy and volume of information in medical research, the significance of comprehensive CDM systems cannot be emphasized enough. To handle the complex characteristics of medical information, multiple tools and technologies have been created to simplify the procedure.
Electronic Data Capture (EDC) systems are leading the way in this technological change, enabling the gathering and storage of medical information in a digital format. This move towards digitization not only replaces outdated paper-based methods but also provides a longitudinal medical history of patients, which is essential for reducing redundancies in care and testing. EDC systems, when implemented effectively, ensure that information from medical studies is collected in a compliant manner, in line with regulatory standards such as ISO 14155:2020, and can significantly enhance the efficiency of information management.
Moreover, Clinical Data Repositories (CDRs) have become a staple in the realm of CDM, providing real-time, patient-centered databases that support the quick retrieval of clinical data. CDRs contain a wealth of information including individual demographics, medication lists, lab results, allergies, procedures, diagnosis, and scheduling information. The intelligent algorithms integrated within CDRs allow for advanced prediction and risk modeling, a feature that is becoming increasingly valuable in patient care.
In addition to these, information management software, validation tools, and reporting systems are essential components of the CDM ecosystem. These technologies automate information input, validate the information to ensure accuracy, and generate comprehensive reports for analysis. These reports aid in decision-making and are essential for drawing scientifically valid and robust conclusions from trials.
It's clear that effective management of accurate medical information goes beyond handling rows and columns of information; it's about guaranteeing the rights, safety, dignity, and well-being of trial subjects are safeguarded. As such, the WHO emphasizes the significance of carefully managing information from clinical studies, as the devices tested could potentially be used by millions of patients.
Given the changing nature of CDM, the healthcare sector should give importance to creating and applying comprehensive resources that effectively navigate and extract value from the abundance of health information. The challenge lies not only in gathering information but also in interpreting it to find clinically relevant information. The primary aim of these technologies is to enhance usability, safety, and satisfaction in the handling of medical information, thereby ensuring the reliability of trial outcomes and supporting regulatory submissions.
Best Practices in Clinical Data Management
Clinical information management is a crucial component in the research process, guaranteeing the precision and dependability of information gathered during clinical trials. To enhance the efficiency of this process, it is vital to adopt standardized collection forms and protocols. These standards facilitate the smooth aggregation and analysis of information from various sources, such as hospitals, health information exchanges, and laboratories. Regular quality checks and audits are essential, improving the integrity and consistency of the information. Furthermore, the safety and confidentiality of clinical information must adhere to strict guidelines to safeguard personal details.
A Clinical Data Repository (CDR) demonstrates an efficient system for organizing and providing real-time, patient-centered information. It functions as a comprehensive database that aids healthcare providers by offering immediate access to vital medical records, thus preventing redundant testing and facilitating predictive modeling through advanced algorithms.
The incorporation of artificial intelligence in healthcare, as discussed at WEDI's Spring Conference, is transforming risk stratification and care for individuals. For example, machine learning tools are now accurately predicting results of spine surgeries, indicating a significant advancement in information usage.
Clinical information control stretches out past the limits of individual trials, affecting the safety of participants and the wider patient population. As per the WHO, with millions of medical devices in use, the careful gathering and handling of study information is crucial. This is emphasized by the European Union Medical Device Regulation, which highlights the requirement for thorough information handling in trials to guarantee participant safety and the accuracy of research results.
In brief, compliance with these recommended methods in managing medical information is not only a procedural requirement but also a dedication to ensuring the safety of individuals and the reliability of research results.
Benefits of Effective Clinical Data Management
The scenery of medical investigation is being deeply changed by the combination of health information repositories (CDR) and artificial intelligence (AI), indicating a fresh period of improved attention to individuals. CDRs serve as real-time, patient-centered databases that not only streamline the retrieval of medical information but also offer a comprehensive view of a patient's medical history, including past procedures and test results. This approach mitigates redundant testing and inefficiencies in care, ultimately supporting healthcare providers in making informed treatment decisions.
An example of the power of efficient information management is the case of hypertension, a leading risk factor for cardiovascular disease affecting over 100 million U.S. adults. With a plethora of treatment options and the impracticality of comparing every possible drug combination through clinical trials, high-quality clinical information becomes a linchpin for identifying optimal treatment strategies. As Dr. Yuan Lu, an assistant professor of medicine, highlights, the overwhelming variety of available medications necessitates a data-driven approach to close the gap in evidence and guide healthcare decisions.
Furthermore, the ability of AI to transform the delivery of medical care depends on the existence of standardized and easily accessible health information. The capacity of AI to improve health outcomes, enhance patient safety, and offer cost-effective, high-quality care is unprecedented. However, progress is hindered by the current lack of standardization in health information, which is essential for training, testing, and monitoring AI tools. This challenge highlights the urgent requirement for strong practices in handling and organizing medical information.
According to the SPIRIT-AI and CONSORT-AI Working Group, the integration of AI, supported by timely and accurate information, is essential for constructing an infrastructure that can cater to an aging population, promote precise treatments, and tackle medical professional burnout. Despite variable progress in individual countries, the global health community recognizes the urgency of monitoring health AI tools for sustained performance as they are implemented across diverse populations and over time.
In the realm of medical technology (MedTech), where trials are paramount, the handling of trial information goes beyond mere points. As mentioned in the European Union Medical Device Regulation, the safeguarding of participant rights, safety, dignity, and the scientific validity of research information are of utmost importance. From a business standpoint, medical information serves as the basis for regulatory body submissions, showcasing the safety and effectiveness of devices meant for market release. In the realm of MedTech, thorough handling of patient information is crucial in guaranteeing participant safety and facilitating accurate deductions from study records.
To summarize, efficient handling of patient information is a fundamental aspect of medical investigation, enabling well-informed decision-making, improving the excellence and reliability of information, and opening doors for the successful incorporation of AI in the healthcare sector. It empowers researchers to discern trends, foster collaboration, and ultimately contribute to the evolution of medical knowledge and treatment modalities.
Case Study: Implementing Integrated Clinical Data Management Systems
The adoption of an integrated clinical information management system by a medical research institute has marked a significant advancement in how clinical information is handled. The system's ability to consolidate information from diverse sources and apply advanced analytics tools has been crucial in unearthing novel insights and patterns within research findings. This groundbreaking approach not only improves the quality of the information but also enhances the efficiency of the research process. The institute is now among the many healthcare entities recognizing the pivotal role of such integrated systems in fostering a data-driven environment that can potentially transform patient outcomes.
Given the intricate nature of healthcare information, the merging of real-world sources with trial details is crucial. Clinical trials that typically function in isolation, separated from wider health information ecosystems, can greatly benefit from integrating information from electronic health records and patient-reported details. This expanded approach contributes to a more comprehensive understanding of patient health, including social determinants and behaviors that occur outside clinical settings. By capturing these numerous information points, researchers can enhance the relevance and impact of their studies, addressing the critical 60-30-10 challenge described by Paige McDonald and colleagues, where a significant portion of healthcare does not align with evidence-based guidelines.
The case study showed that when such integrated information management systems are implemented, they pave the way for a more nuanced and complete picture of health. This is echoed by the analogy of a painter who, with a broader palette of colors, can create more nuanced and elegant art. Similarly, a researcher equipped with a diverse array of information can conduct analyses that are more pertinent to a wide population. The potential for such systems to streamline the process of clinical trials was also highlighted, noting the drawbacks of traditional information collection methods that often neglect long-term insights and contextual information about trial participants.
Furthermore, the necessity for shared health information across international borders, as highlighted during the COVID-19 outbreak, emphasizes the importance of global interoperability. The digitization of health records and the push towards standardized information elements have been supported by public investment globally, which is crucial for the future of healthcare research and delivery.
The importance of a comprehensive repository of medical records cannot be emphasized enough—it enables the timely access to an individual's medical background, supports predictive modeling, and reduces unnecessary testing and care inefficiencies. Advocates for effective handling of medical information emphasize the importance of safeguarding participant safety and ensuring the scientific validity of trials, which is crucial for regulatory submissions and the commercial success of medical devices. Basically, the case study acts as evidence of how combining medical information can enhance investigation capabilities and ultimately enhance the quality of care for individuals.
Future Trends in Clinical Data Management: AI and ML Integration
The healthcare sector is currently undergoing a significant transformation with the integration of artificial intelligence (AI) and machine learning (ML), which are set to revolutionize clinical information management. State-of-the-art AI algorithms can now perform real-time analysis, like tracking vital signs, electronic health records, and laboratory outcomes, to anticipate the decline in health of individuals. This ongoing monitoring, which operates in the background and evaluates patient information approximately every 15 minutes, enables the determination of risk scores and timely alerts to the care team if a patient's condition is deteriorating.
For example, an AI-driven deterioration model running in a hospital setting exemplifies how AI can create more resilient health systems, particularly in high-stakes situations. The model is not just a technological advancement; it also facilitates crucial conversations and interventions that may otherwise not occur, thus strengthening the communication channels within the healthcare system. It also addresses the problem of sepsis, which has been recognized as a critical issue that can be more effectively managed with the support of a specially developed AI tool.
The growing amount of healthcare information is another aspect where AI and ML are making a significant impact. Roughly 30% of the world's information volume is produced by the healthcare sector, and this is projected to increase at a compound annual growth rate of 36% by 2025. This rapid growth requires the creation of versatile tools that can effectively navigate and interpret this abundance of health information. Ai's capacity to sift through the 'data dumpsters' of legacy electronic health records (EHRs) is a game-changer, providing clinicians with access to pertinent information that would otherwise be buried in free-text notes and disparate data sources.
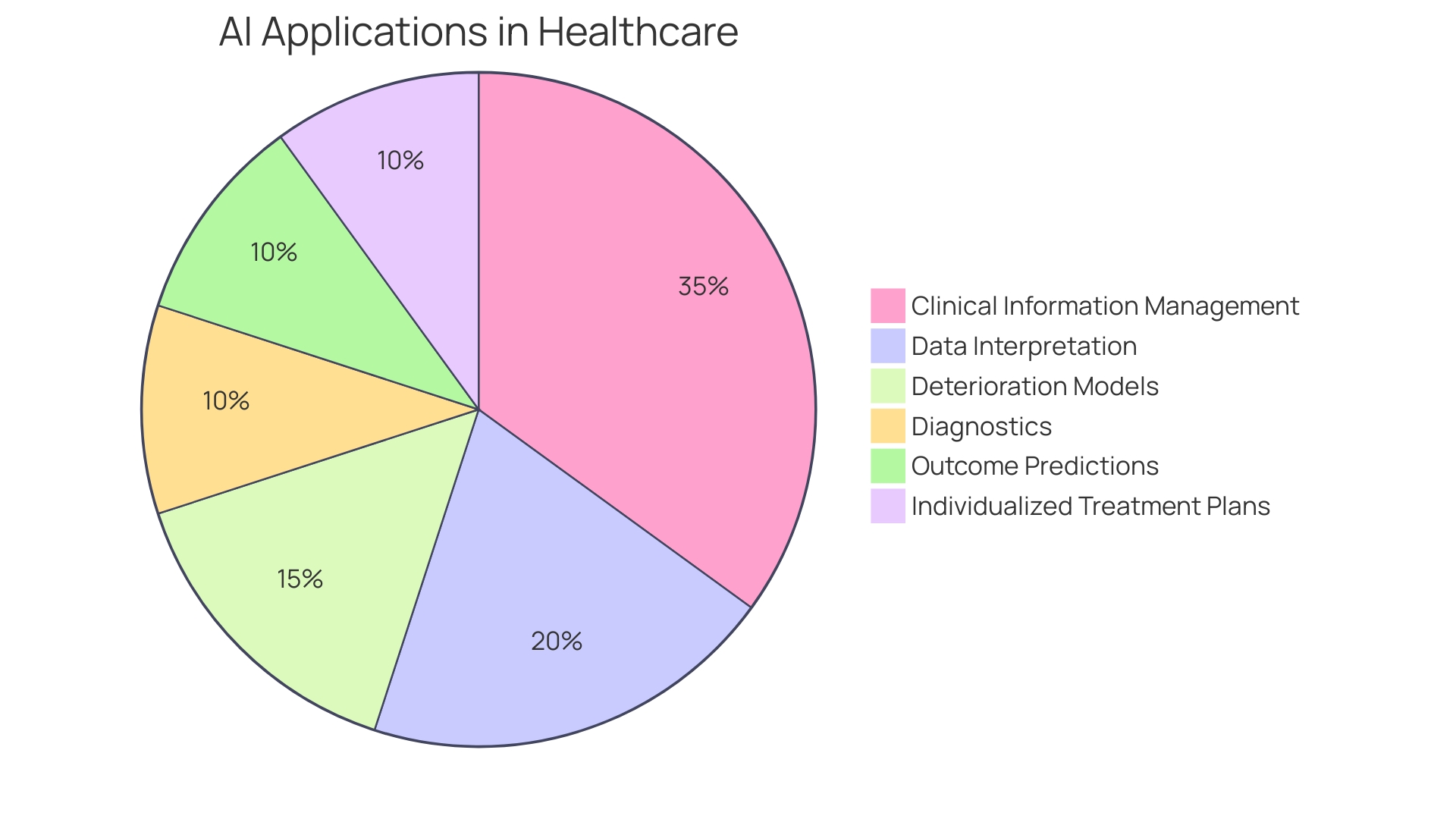
The market size of AI in healthcare reflects the increasing demand for such transformative technologies. Health systems are recognizing the high potential impact of AI investments, particularly in areas like virtual health, digital front doors, and operational efficiency. Despite some health systems not planning immediate investment, the recognition of Ai's potential suggests that future integration is inevitable. This sentiment is supported by the historical path of AI in healthcare, from its conceptual origins to the current advanced applications that aid in diagnostics, outcome predictions, and the individualization of treatment plans.
Investments in AI are also evident in the actions of leading institutions. For example, Mayo Clinic's use of a $25 million donation for AI-related projects, the establishment of the Cancer AI Alliance through a $40 million tech company funding, and the development of SeptiBurnAlert by US Army researchers, an AI system that predicts sepsis risk in burn individuals, all demonstrate the dedication to advancing AI in healthcare. These initiatives, among others, herald a future where AI and ML are not just auxiliary tools but integral to the fabric of clinical data management and patient care.
Conclusion
In conclusion, clinical data management is crucial for ensuring the integrity and reliability of data collected during clinical trials. It plays a vital role in the success of medical research and the approval of medical devices. Standardization and interoperability enhance the validity of study findings, while robust security measures protect patient information.
Data managers are pivotal in orchestrating the data lifecycle, and Clinical Data Repositories provide real-time access to comprehensive clinical data. Various tools and technologies streamline the data collection and analysis process.
Adhering to best practices, including standardized data collection and regular quality checks, ensures data accuracy and reliability. Effective clinical data management brings benefits such as enhanced patient care, improved research outcomes, and the integration of AI in healthcare.
The integration of AI and ML is a future trend that can revolutionize patient care. AI algorithms enable real-time data analysis and continuous monitoring for predicting health deterioration. Investments in AI in healthcare reflect its potential impact on diagnostics, patient outcomes, and treatment personalization.
In summary, effective clinical data management is essential for successful medical research and the delivery of safe and effective patient care. It requires standardized processes, robust security measures, and a commitment to data integrity. The integration of AI and ML further enhances the potential of clinical data management in shaping the future of healthcare.




