Introduction
The integration of Electronic Data Capture (EDC) systems in clinical research has revolutionized the way data is collected and managed. Moving away from traditional paper-based methods, EDC systems offer a streamlined and digital approach to gathering patient data. These systems not only enhance the accuracy and integrity of clinical trial data but also provide easier access and analysis.
With the ability to capture real-time data and securely store it in an electronic database, EDC systems mitigate issues such as missing or ambiguous entries often associated with paper methods. The utilization of EDC is further exemplified in 'EHR-sourced' trials, where patient data sourced from Electronic Health Records (EHR) is leveraged for research purposes. The ambition of digitizing health and social care records by the National Health Service (NHS) further underlines the significance of EDC systems in modern healthcare.
Investments in cloud-based solutions and interoperable products are poised to revolutionize the field, enabling healthcare professionals to access and utilize digital health data more effectively. As the healthcare industry continues to embrace digital solutions, EDC systems will play a pivotal role in ensuring the integrity and accessibility of clinical research data.
What is Clinical Electronic Data Capture (EDC)?
The integration of Electronic Data Capture (EDC) systems in clinical research has marked a critical shift from cumbersome paper-based data collection to a more streamlined, digital approach. EDC systems, which efficiently gather and manage patient data in electronic form, are not only enhancing the accuracy and integrity of clinical trial data but are also facilitating easier access and analysis. By directly capturing data from participants or healthcare providers, EDC systems ensure that data is recorded in real-time and stored securely in an electronic database, mitigating issues such as missing or ambiguous entries commonly associated with paper methods.
The utilization of EDC is further exemplified in 'EHR-sourced' trials, where patient data sourced from Electronic Health Records (EHR) is leveraged for research purposes. A study highlighted in Trials journal elucidates the demonstration of a project where EHR data complemented data collected for a multi-center pharmaceutical industry outcomes trial. It underscored how a central coordinating center was instrumental in addressing the technical, governance, and operational challenges of integrating EHR data into clinical research, thereby enhancing data quality and integrity.
Moreover, the ambition of the National Health Service (NHS) to digitize the majority of health and social care records by March 2025, as part of the Department of Health and Social Care’s digital plan, underpins the importance of EDC systems in modern healthcare. This digital transformation is anticipated to level up the digital maturity across healthcare foundations, a move celebrated by industry leaders and healthcare providers alike. Investments in cloud-based solutions and interoperable products are poised to further revolutionize the field, enabling healthcare professionals to access and utilize digital health data more effectively.
The advent of technology has made it possible to capture a wealth of data from a variety of sources, including connected devices and wearables, which can provide deeper insights and inform decisions in drug development. However, managing and analyzing the vast volumes of data necessitates a robust data strategy that is established before the trial protocol is designed. This strategy should consider how traditional and digital data sources will be integrated to optimize data collection and flow throughout the clinical trial process.
With the potential to improve the quality of healthcare and reduce costs, the EHR represents a significant advancement in healthcare service delivery. When health data is shared electronically, it not only streamlines communication of patient information but also supports the continuous, efficient, and quality delivery of health services on a global scale. As the healthcare industry continues to embrace digital solutions, EDC systems will play a pivotal role in ensuring the integrity and accessibility of clinical research data.
Benefits of Using EDC in Medical Research
Electronic Data Capture (EDC) systems have revolutionized the field of medical research by enhancing the efficiency and accuracy of data management. Transitioning from traditional paper-based methods, EDC systems play an integral role in streamlining the data collection process. This advancement is especially critical in time-sensitive medical scenarios, such as when evaluating potential heart attack cases.
Dr. Brian Patterson from the University of Wisconsin-Madison emphasizes the urgency in such situations, stating, 'For individuals who may be having a heart attack, every moment counts.' The introduction of EDC systems allows for real-time data analysis, which is crucial in reducing the delay in medical intervention that can result from the laborious process of blood sample laboratory analysis—a method still deemed the gold standard in diagnosing heart attacks despite its time constraints.
The utility of EDC systems extends to the monitoring of new medical treatments, as seen in the University of California Health's Center for Data-driven Insights and Innovation (CDI2) which employs real clinical data to expedite the discovery of effective medications. This approach not only saves time but also significantly cuts down on research costs. Similarly, a study by the University of Nottingham utilizes EDC technology for a clinical evaluation involving adult participants, highlighting the myriad of benefits it offers, including the potential elimination of additional monitoring devices.
In the realm of medical data entry, which often requires substantial resources due to its volume and complexity, EDC systems offer a solution by outsourcing data processing to third-party vendors. This shift not only improves data quality through built-in validation checks but also unburdens medical staff from time-consuming tasks, allowing them to focus on more critical aspects of patient care.
Moreover, the design of controlled medical research studies is greatly facilitated by EDC systems. They provide a robust framework for collecting and managing data, which is essential for maintaining the integrity of the study. As researchers manipulate independent variables and measure outcomes, the EDC systems ensure that data is captured accurately and systematically, minimizing biases and confounding factors.
The impact of EDC systems on healthcare data management is further supported by statistics that highlight the advantages of electronic reporting. These systems enable the extraction of comprehensive data sets that can inform future measure development and support the practical use of EHRs in clinical trial settings. The significance of such technological advancements is underscored by the increasing need for efficient diagnostic tools in emergency departments, which directly influence patient care outcomes.
Overall, EDC systems represent a significant leap forward in the digitization of healthcare data, offering a powerful tool that aligns with the urgent need for rapid and reliable medical research methodologies.
Key Components of an EDC System
Electronic Data Capture (EDC) systems are integral to modern clinical research, providing a robust infrastructure for data management. These systems are equipped with a user-friendly interface that facilitates seamless data entry, and a secure database where this information is meticulously stored. To guarantee the accuracy and reliability of data, EDC systems incorporate validation rules that scrutinize the data for quality assurance.
A suite of analytical tools is also available within these systems, enabling researchers to efficiently analyze and interpret the vast quantities of data collected during clinical trials.
Furthermore, EDC systems offer advanced features to enhance data security and ensure compliance with stringent regulatory standards. These features include electronic signatures, which validate the authenticity of the data, audit trails that track data changes over time, and role-based access controls that safeguard sensitive information by restricting system access to authorized personnel only.
The digital revolution, particularly in healthcare, underscores the importance of such systems. With the advent of electronic health records (EHR), the industry has seen a significant shift from traditional paper-based records to more dynamic, accessible, and comprehensive digital records that track a patient's healthcare journey in real time. This transition not only streamlines the process of healthcare delivery but also improves the quality of patient care.
Moreover, as clinical research grows more sophisticated with the inclusion of connected devices and decentralized trial solutions, the volume and variety of data that EDC systems need to handle have increased dramatically. Artificial intelligence-driven methodologies are now essential to draw meaningful insights from this data, making it imperative for EDC systems to be robust, scalable, and strategically aligned with trial goals.
In practice, the use of EDC systems has demonstrated substantial improvements in data quality and integrity. For example, the transition from paper diaries to electronic systems has addressed issues such as missing or ambiguous data entries, which were prevalent when data were manually recorded. This has been evidenced by studies that reveal a significant reduction in data entry errors when utilizing electronic methods.
The evolution of EDC systems continues to play a pivotal role in advancing clinical research, ensuring that the data collected is of the highest quality, securely managed, and effectively utilized to support the development of new medical treatments and therapies that ultimately benefit patients worldwide.
Steps to Implement an EDC System in Clinical Trials
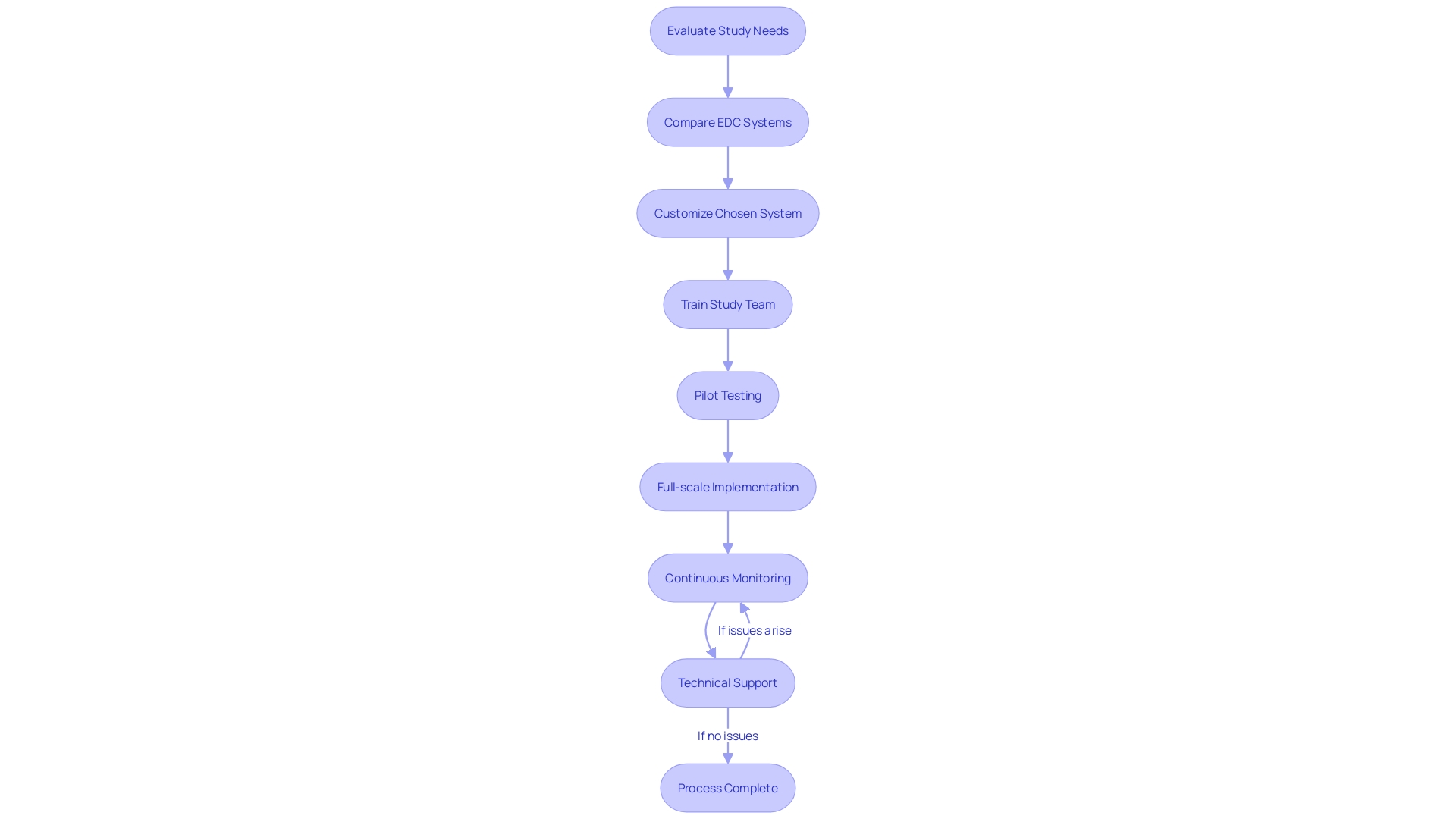
The integration of an Electronic Data Capture (EDC) system into clinical research is a multi-faceted process that demands attention to detail to enhance the study's data collection and management capabilities. Initially, it is imperative to evaluate the study's unique needs and determine the essential features of the EDC system that align with the study's objectives. Subsequently, a thorough comparison of the various EDC systems on the market should be conducted to select the most suitable one.
Once an EDC system is chosen, it necessitates customization to adhere to the study's protocol, which includes setting up data entry forms, defining validation rules, and establishing user roles. The study team must then undergo extensive training to become proficient with the EDC system's functionalities.
Before full-scale implementation, it is beneficial to engage in pilot testing to evaluate the EDC system's performance and user-friendliness. Following successful customization and testing, the EDC system can be deployed across all study sites, ensuring seamless data migration and integration with other systems involved in the study.
Continuous monitoring of the EDC system is essential, along with providing consistent technical support to swiftly address any operational challenges that may arise. This vigilant approach to implementing and supporting an EDC system is pivotal for the integrity and success of clinical research.
Reflecting on a recent demonstration project highlighted in Trials journal, which utilized EHR data to supplement a multi-center pharmaceutical industry outcomes trial, it is evident that central coordination is vital in navigating the technical, governance, and operational intricacies of such activities. This project, while conducted within a large integrated health system in the United States, offers valuable insights applicable to health systems of all sizes, emphasizing the potential of clinical text processing to enhance healthcare delivery and quality.
Moreover, as per the Editor in Chief of the Journal of Clinical and Translational Science, the burgeoning field of decentralized clinical trials underscores the importance of developing standardized best practices to ensure the generation of quality results. With the decentralized clinical trial market projected to grow at a CAGR of 30.1% from 2021 to 2026, addressing challenges such as data security, privacy, and technological accessibility becomes increasingly pertinent.
In conclusion, the implementation of EDC systems in clinical trials is a strategic endeavor that should be executed with precision and foresight, considering the evolving landscape of clinical research and the expanding scope of electronic health records in trial settings.
Best Practices for Data Management with EDC
To optimize the use of Electronic Data Capture (EDC) systems in medical research, it's essential to adopt a series of well-defined data management practices. Firstly, standardizing data collection methods across various study sites fosters uniformity and enables consistent data comparison. Data validation checks within EDC systems are pivotal for real-time identification and prevention of entry errors.
It's also crucial to have robust data quality control processes in place for regular review and cleansing of data to rectify any inconsistencies.
Securing sensitive information is of utmost importance; hence, implementing stringent security protocols such as user authentication, data encryption, and access controls is necessary. Along with security, maintaining routine data backups and having a comprehensive disaster recovery plan ensures the preservation and restoration of data in case of unforeseen events.
Monitoring data entries and conducting periodic audits are essential to adhere to data quality standards and meet regulatory compliance. These audits also help in maintaining the credibility and integrity of the research data.
In line with these practices, recent studies such as Raman et al. Trials (2023) 24:566, demonstrate the integration of EDC and electronic health record (EHR) data to enhance data quality and operational efficiency in multicenter trials. Additionally, the recent release of a comprehensive surgical database by UCI Medical Center highlights the growing emphasis on data diversity and the potential of EDC systems to support advanced research, such as the development of AI algorithms for clinical decision support.
These examples underscore the importance of meticulous planning and execution in data strategy to handle the exponential growth in data volume from diverse sources, including traditional and digital means. By implementing these data management best practices, we can ensure that the wealth of information collected through clinical trials is harnessed effectively to advance patient-centric drug development and improve healthcare outcomes globally.
Regulatory Considerations for EDC Systems
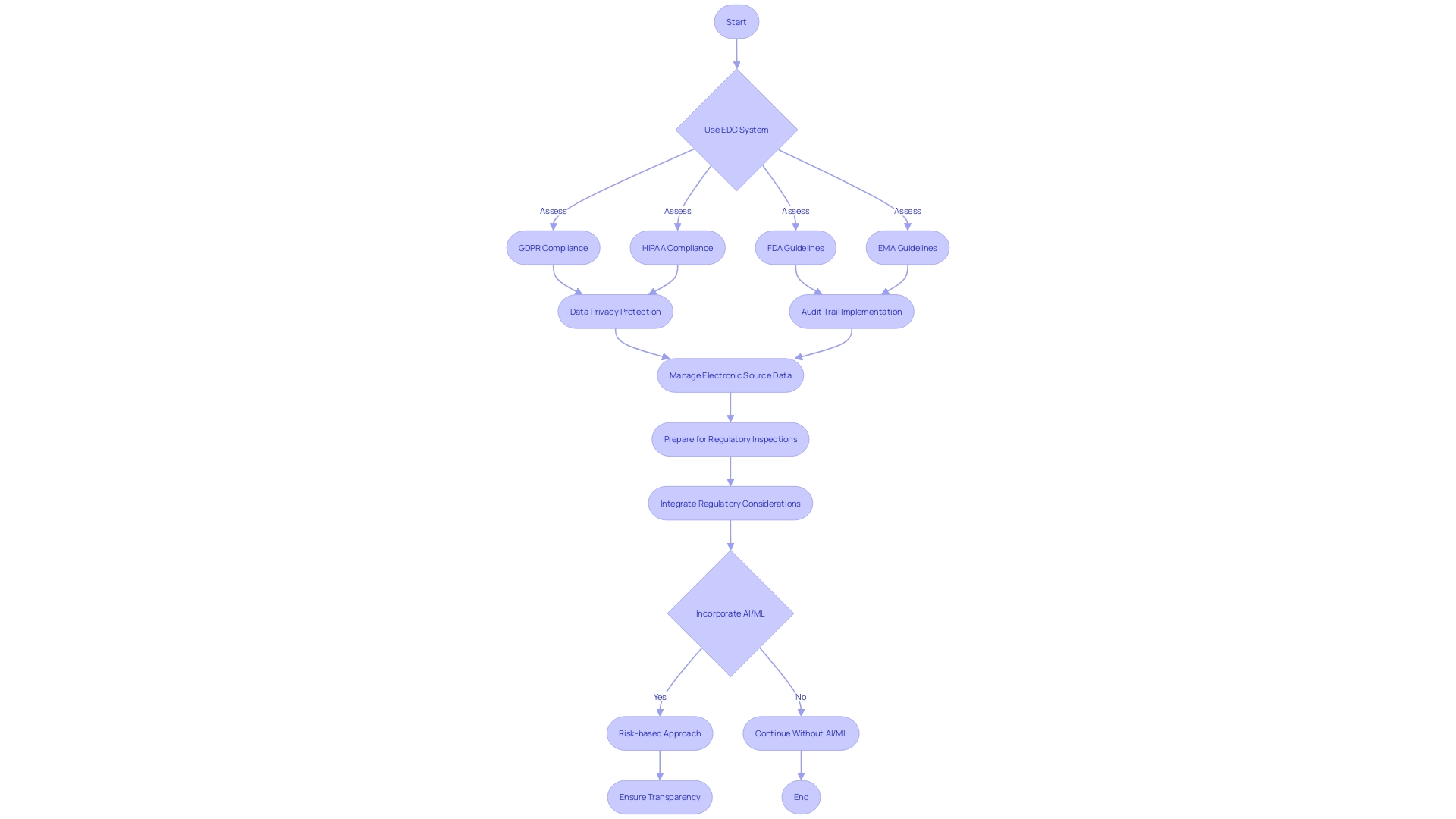
Ensuring regulatory compliance in the use of Electronic Data Capture (EDC) systems during clinical trials is paramount. To maintain adherence to regulations such as the GDPR and HIPAA, it is crucial to confirm that the EDC system is equipped to protect data privacy. Additionally, adherence to guidelines from FDA and EMA regarding electronic records and signatures is non-negotiable.
An effective audit trail within the EDC system is essential to preserve data integrity and provide a transparent record of all data modifications. This capability is critical to uphold the trustworthiness of the data, especially in the context of EHR-sourced trials where patient clinical data is utilized.
The management of electronic source data also demands rigorous processes to ensure it satisfies regulatory standards for use in submissions. This aspect of data management is becoming increasingly relevant as the trend towards decentralized clinical trials (DCTs) grows, with a projected CAGR of 30.1% from 2021 to 2026, emphasizing the necessity for standardized and validated systems.
Lastly, preparation for regulatory inspections should be thorough, with comprehensive documentation, data management protocols, and security measures fulfilling regulatory expectations. This preparedness is especially pertinent given the rapid evolution of regulations, such as those concerning medical devices and Software as a Medical Device (SaMD), which are experiencing significant expansion with a CAGR of approximately 52% from 2023 to 2028.
The integration of these considerations into clinical trial design and execution is critical to ensure quality results and the successful operationalization of study goals, as demonstrated in a project that utilized EHR data to supplement a multi-center pharmaceutical industry outcomes trial. The project showcased how a central coordinating center can effectively support trial sites through technical, governance, and operational challenges, thereby enhancing the practical utilization of EHRs in clinical research.
Case Studies: Successful Implementation of EDC in Medical Research
Recent case studies have shed light on the transformative role of Electronic Data Capture (EDC) systems in the realm of medical research, particularly in studies utilizing electronic health records (EHR). A demonstration project, as cited in Trials (2023) 24:566, offers a comprehensive illustration of EDC's efficacy. This project adeptly integrated EHR data with data collected from a multi-center pharmaceutical industry trial, efficiently bridging technical, governance, and operational facets.
The study meticulously navigated site selection, data extraction strategies, and the meticulous process of data transfer and quality review, revealing operational intricacies and imparting valuable lessons learned. It underscored how a well-orchestrated central coordinating center can bolster the capabilities of participating sites, ensuring high-quality data management and contributing to safer patient outcomes and expedited study timelines.
In parallel, advancements in artificial intelligence (AI) are further revolutionizing data analysis in clinical research. For instance, Scripps Research developed an innovative AI tool that can reconstruct comprehensive 12-lead ECG data from limited electrode inputs, as reported by Giorgio Quer, PhD. This significant leap in data processing capability exemplifies the potential to enhance data analysis, leading to more nuanced and precise medical insights.
Despite the successes, the journey towards seamless integration of EDC systems is not without its challenges. Data quality issues remain a pivotal concern, with the prevalence of inaccurate data entry, duplicate records, and incomplete data posing significant threats to the integrity of clinical research. Addressing these challenges requires a concerted effort to ensure data diversity, security, privacy, and interoperability, which are essential for maintaining the robustness of clinical trial data.
As the industry continues to evolve, the focus on these operational and technical considerations will be critical in maximizing the potential of EDC systems in improving the efficiency and outcomes of clinical research.
Common Challenges and Solutions in EDC Implementation
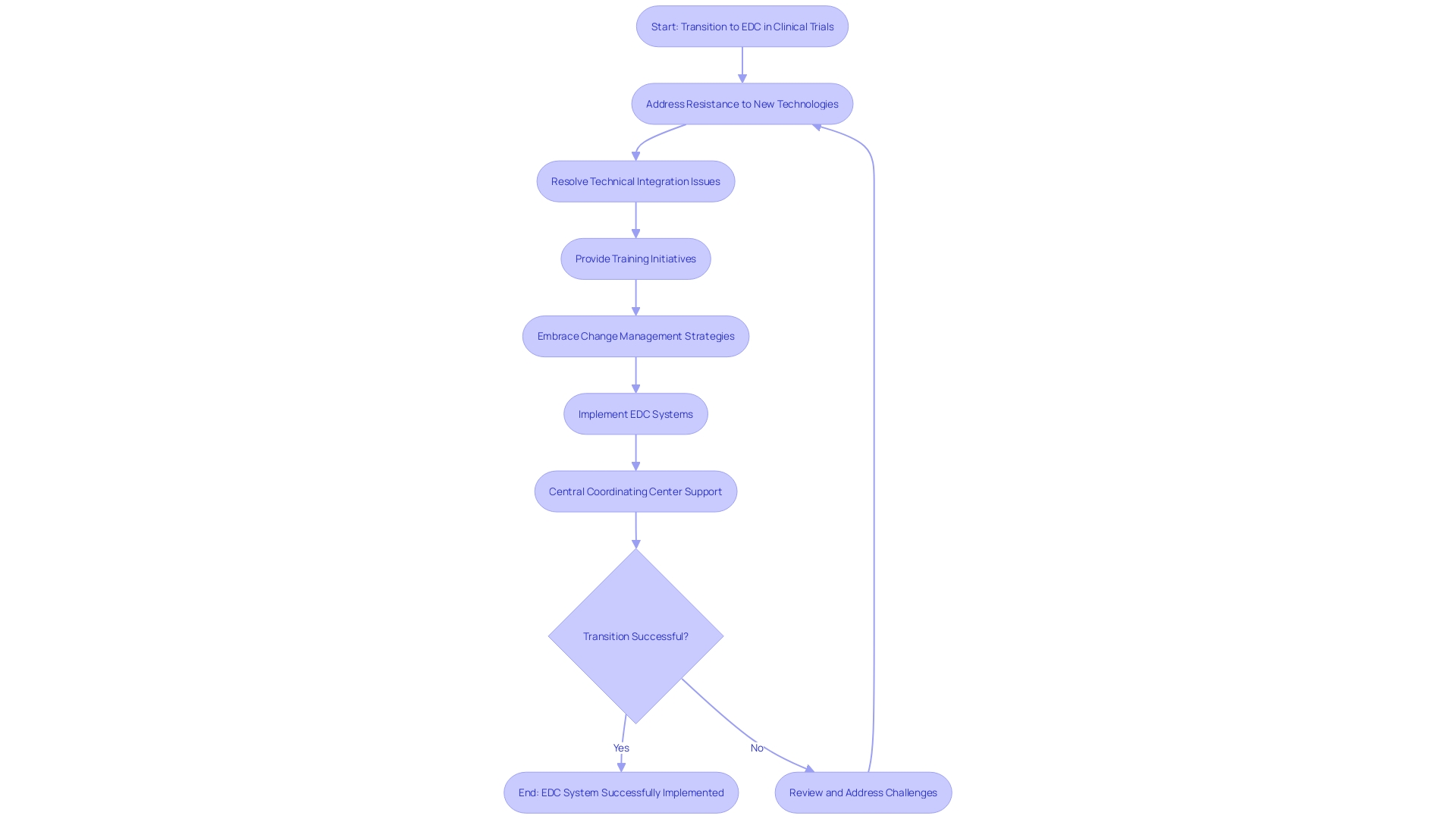
Electronic Data Capture (EDC) systems are revolutionizing clinical trials by enhancing data accuracy and accessibility. However, the transition from traditional paper-based methods to sophisticated EDC platforms is not without its hurdles. Resistance to adopting new technologies can be prevalent among study teams, and integrating EDC systems with existing infrastructure often presents technical challenges.
Moreover, the study team may require extensive training to proficiently use these new systems. Addressing these issues demands a proactive approach, including comprehensive change management strategies to ease the transition, technical support to resolve integration issues, and ongoing training initiatives to build team competency. By embracing such measures, clinical trials can harness the full potential of EDC systems, ensuring data integrity and streamlining research processes.
A central coordinating center can play a pivotal role in this transition, as demonstrated in a project where EDC data complemented traditional data collection methods, reinforcing the operational, governance, and technical support to participating sites. Such advancements are crucial, especially as the decentralized clinical trial market is set to expand significantly, with a projected compound annual growth rate of 30.1% from 2021 to 2026. These decentralized trials highlight the need to meet participants 'where they are,' thereby addressing accessibility issues and expanding the potential participant pool to include those in remote or underserved regions.
The success of EDC implementation and the growth of decentralized trials underscore the evolving landscape of clinical research, which aims to bring trials closer to patients while ensuring the highest standards of data quality and regulatory compliance.
Future Trends in EDC Technology
Evolving EDC technology is transforming the landscape of clinical research, with innovative trends poised to enhance data collection and analysis. Mobile EDC solutions are at the forefront, leveraging smartphones and tablets for more flexible and convenient data entry. The integration with wearable devices and sensors represents another advancement, offering real-time, continuous patient monitoring for a richer data set.
Artificial intelligence and machine learning are revolutionizing data handling, streamlining validation, and extracting patterns and insights from extensive datasets. In tandem, blockchain technology promises to bolster EDC systems with unmatched data security, transparency, and integrity.
Furthermore, the shift towards remote monitoring and virtual trials is underway, aiming to minimize the need for physical visits while improving patient engagement and retention. These trends are not merely speculative; they reflect a broader movement towards digitization in healthcare, as evidenced by emerging technologies like the Infrasensor wristband. This device utilizes infrared light to non-invasively measure cardiac biomarkers such as troponin, expediting the diagnosis of heart attacks, which is critical given that over 800,000 occur annually in the U.S. alone.
The digitization of medical devices is inevitable, bringing with it the promise of preventive medicine and enhanced patient care through IoMT (Internet of Medical Things), which facilitates the secure transmission of medical data.
Yet, with this digital evolution comes the paramount concern of data privacy and cybersecurity. As the reliance on medical AI and ML grows for tasks such as rapid diagnostics and robotic surgery, the industry must navigate the dual challenges of maximizing the potential of these technologies while safeguarding against data breaches and unauthorized access.
Overall, the progress in healthcare technology and innovation is accelerating, with a clear demand for advanced medical devices, wearables, and improved health services. However, the challenges of data security and patient privacy persist, requiring a delicate balance to ensure the benefits are realized without compromising patient trust and safety.
Conclusion
In conclusion, the integration of Electronic Data Capture (EDC) systems in clinical research has revolutionized data collection and management. EDC systems streamline and digitize the process, enhancing accuracy, integrity, and accessibility of clinical trial data. The utilization of EDC in 'EHR-sourced' trials leverages patient data from Electronic Health Records for research purposes.
The ambition of the NHS to digitize health and social care records underscores the significance of EDC systems in modern healthcare. Investments in cloud-based solutions and interoperable products further revolutionize the field, enabling effective utilization of digital health data.
EDC systems provide numerous benefits in medical research, including efficiency, accuracy, and monitoring of new treatments. They offer a robust infrastructure for data management, incorporating validation rules, analytical tools, and advanced features for security and compliance.
Implementing an EDC system in clinical trials requires careful evaluation, customization, training, and pilot testing. Continuous monitoring and technical support are essential for successful implementation. Best practices for data management with EDC include standardization, validation checks, data security, and regular audits.
In summary, EDC systems play a pivotal role in ensuring the integrity and accessibility of clinical research data, supporting the advancement of medical treatments. The ongoing evolution of EDC technology and adoption of best practices will continue to drive improvements in data management and research outcomes.




