Introduction
Clinical research consulting firms play a crucial role in the successful execution of complex clinical trials. With the intricacy of these trials and the rigorous regulatory environment, pharmaceutical companies are increasingly relying on specialized expertise to guide them through the process. These firms provide comprehensive services and leverage cutting-edge technology to optimize trial design and execution, ultimately improving patient outcomes.
In this article, we will explore the importance of clinical research consulting, the methods they employ, and the results they achieve in enhancing trial processes and addressing challenges. We will also delve into case studies that highlight the transformative potential of these firms in advancing medical research and accelerating the journey from trial to treatment.
Background
The intricacy of clinical trials and the rigorous regulatory environment necessitate specialized expertise to ensure their successful execution. Clinical research consulting firms are increasingly sought after by pharmaceutical companies to guide these complex processes. For instance, Treehill Partners, a transaction advisory firm with over two decades of experience, recognized a pattern where companies regretted certain decisions made in earlier phases of their studies.
As articulated by a Tree hill advisor, an analysis of 1,200 data points revealed that approximately 80% of decisions made three to four years prior to a study's completion could have been improved with more thorough planning and consulting. This insight has driven Tree hill to optimize their consulting services, likening the process to strengthening the links in a chain to improve the overall outcome.
Similarly, CMIC Group, Japan's pioneering CRO, emphasizes the importance of tailored solutions that span the entire pharmaceutical value-chain. Their commitment to innovation and meeting clients' specific needs has been a game-changer for pharmaceutical companies, medical device manufacturers, and academic institutions.
The partnerships between research institutions and industry are also pivotal. Gabriel Jones, a Principal Investigator at the Nuffield Department of Women's and Reproductive Health, shared his experience of collaborations that have tangibly improved pregnancy outcomes globally.
Amidst these developments, AI is transforming fields like cardiology, where the integration of machine learning with medical devices is enhancing diagnostic accuracy and reducing the pressure on medical professionals. The potential for AI to revolutionize clinical trials and medical research is becoming increasingly evident.
These case studies and advancements underscore the critical role of clinical research consulting in navigating the complexities of clinical trials. By offering comprehensive services and leveraging cutting-edge technology, these firms are instrumental in advancing medical research and ultimately improving patient outcomes.
Methods
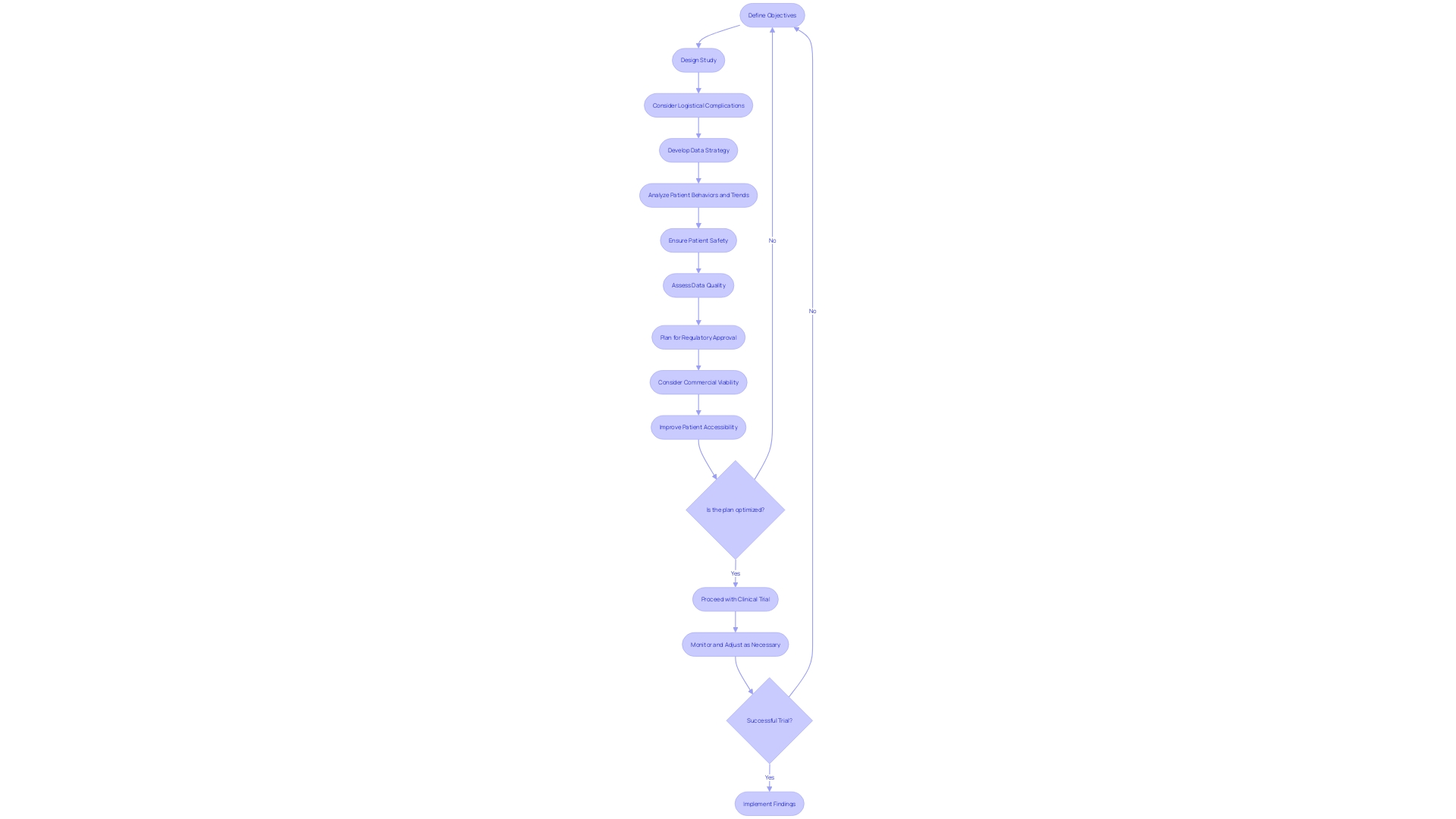
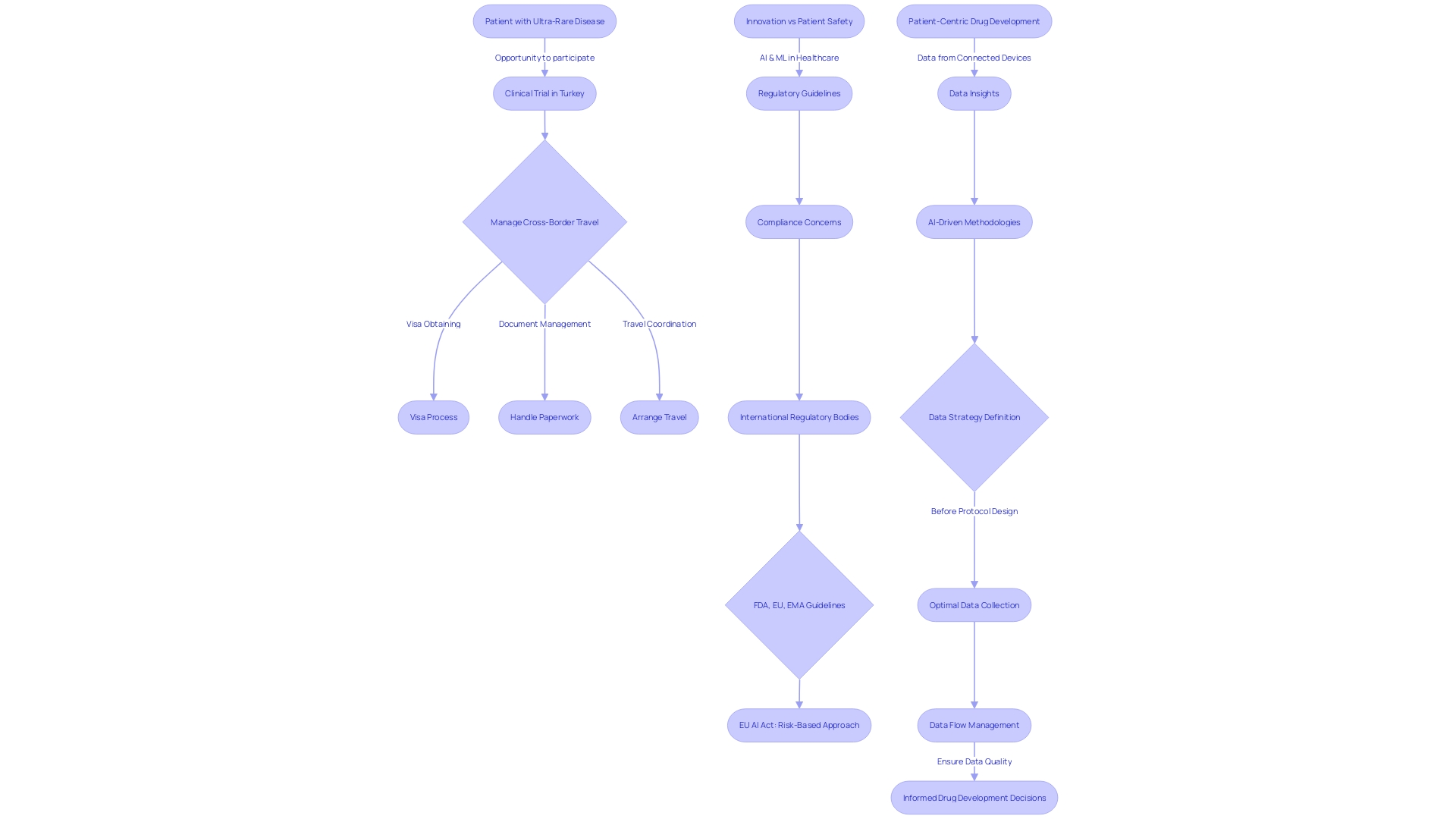
As the clinical trial landscape becomes more intricate and patient-centric, clinical research consulting firms are taking a proactive approach to trial design and execution. They begin with an in-depth analysis of the pharmaceutical company's objectives, study design, and possible logistical complications, such as the case of a patient needing to travel internationally for a trial. Understanding that each trial is a complex chain of decisions, experts emphasize that nearly 80% of these decisions could be optimized with more careful planning and strategic foresight.
For instance, leveraging data insights from various sources, including but not limited to connected devices, wearables, and electronic diaries, can significantly enhance the understanding of patient behaviors and trends. However, this is only possible with a well-defined data strategy that is established before protocol design, ensuring that data is collected efficiently and analyzed comprehensively to support patient safety and data quality.
The evolving nature of clinical trials, highlighted by the shift towards decentralized solutions and the imperative of accelerating drug development, underscores the urgency of meticulous planning. With McKinsey reporting that the average clinical trial duration has increased and up to 80% of trials failing to finish on time, the role of clinical research organizations becomes ever more critical. This is compounded by the pressure to be first to market in a competitive landscape where early market entrants often achieve disproportionate success.
Given the challenges facing the industry, including the vast number of rare diseases with unmet medical needs and the push for patient-centered drug development, clinical research consulting firms are dedicated to ensuring that trials not only meet regulatory approval but also consider the long-term commercial viability and accessibility for patients. This approach is crucial in a healthcare environment where timely and effective treatment is paramount for improving patient outcomes in both common and rare conditions.
Results
Facilitating the execution of complex clinical trials, a consulting firm provided invaluable support to a pharmaceutical company, enhancing their trial processes and addressing challenges. By implementing patient-centric strategies and leveraging connected devices, wearables, and electronic diaries, they optimized patient recruitment and bolstered data collection. This approach led to the integration of AI-driven methodologies that enabled the analysis of a plethora of data points, yielding richer insights into patient behaviors and trends.
The firm's guidance ensured regulatory compliance and facilitated a data strategy that preemptively defined the optimal collection of both traditional and digital data sources. Consequently, the company noted a significant uptick in efficiency, with fewer protocol deviations and higher data integrity contributing to expedited, more precise outcomes.
Abstract
A pivotal case study reveals the significant strides made by a clinical research consulting firm in assisting a pharmaceutical company to bolster its medical research. This firm adeptly navigated the labyrinth of clinical trial complexities, thereby enhancing the trial's efficiency and data integrity, and ultimately contributing to its success. The consulting firm's role was instrumental in harnessing the power of advanced artificial intelligence-driven methodologies to distill actionable insights from diverse data sources, including lab results, patient-reported outcomes, and imaging.
This data-driven approach, which surveyed multiple perspectives and integrated an array of traditional and digital data points, was key in capturing nuanced patient behavior and trends. The case study underscores the transformative potential of clinical research consulting to propel healthcare innovation forward by ensuring that clinical trials are not only compliant with safety and quality standards but also strategically optimized for data collection and analysis.
The strategic foresight applied in the data strategy, initiated prior to protocol design, allowed for efficient data management and real-time risk oversight. Questions surrounding data management expectations, study risks, and the impact of data flow on sites were meticulously addressed by experts within the consulting team, showcasing their deep understanding of the requisites for high-quality digital data integration. The case study is a testament to the critical importance of expert guidance in clinical research, which can lead to significant time and cost savings for pharmaceutical companies by expediting processes such as data submission to regulatory bodies.
With these advancements, the journey from trial to treatment can see a marked reduction in the typical four to six-month waiting period, aligning with industry trends that prioritize patient-centered drug development and the integration of decentralized trial solutions.
Conclusion
Clinical research consulting firms are instrumental in the successful execution of complex clinical trials. They provide specialized expertise and comprehensive services to guide pharmaceutical companies through trial design and execution. By leveraging cutting-edge technology and data insights, these firms optimize patient recruitment, enhance data collection, and ensure regulatory compliance.
The case studies highlighted in this article demonstrate the transformative potential of clinical research consulting. These firms integrate AI-driven methodologies and leverage connected devices, wearables, and electronic diaries to provide richer insights into patient behaviors and trends. They address challenges and optimize decision-making processes throughout the trial journey.
Clinical research consulting firms are dedicated to patient-centered approaches and ensuring long-term commercial viability and accessibility for patients. Their expertise leads to improved trial efficiency, enhanced data integrity, and expedited outcomes. By optimizing data collection and analysis, these firms enable pharmaceutical companies to make informed decisions and accelerate the journey from trial to treatment.
In summary, clinical research consulting firms play a vital role in navigating the complexities of clinical trials. Through their comprehensive services, cutting-edge technology, and strategic planning, they advance medical research and improve patient outcomes. These firms shape the future of healthcare innovation by optimizing trial processes, addressing challenges, and accelerating the journey from trial to treatment.




