Introduction
Clinical research organizations (CROs) have revolutionized the landscape of clinical trials, offering a wide range of services that span the entire pharmaceutical value chain. From trial management to development, manufacturing, and market entry strategies, CROs play a critical role in advancing medical treatments efficiently and effectively. They address logistical challenges faced by patients, facilitate cross-border collaborations, and simplify the process of finding tailored clinical trials.
Furthermore, CROs contribute to the ethical considerations of participant compensation and collaborate with patient organizations to raise awareness about diseases and potential treatments. With their tailored solutions and collaborative spirit, CROs are instrumental in driving the progress of medical research and enhancing patient outcomes.
What are Clinical Research Organizations (CROs)?
Contract Research Organizations (CROs), such as Japan's CMIC Group, have revolutionized the landscape of clinical trials. They offer an extensive range of services that not only assist in trial management and execution but also span the entire pharmaceutical value chain, including development, manufacturing, and market entry strategies. This holistic approach meets the diverse needs of clients, from pharmaceutical companies to medical institutions, ensuring that new medical treatments are advanced efficiently and effectively.
CROs play a critical role in the research ecosystem, especially when addressing logistical challenges faced by patients in clinical trials. For instance, a patient from rural Pennsylvania with a rare disease might have the chance to join a trial in Turkey. This scenario raises numerous logistical questions from obtaining visas to handling foreign paperwork, highlighting the need for comprehensive support systems.
CROs can be pivotal in facilitating such cross-border collaborations, ensuring that patients can access potentially lifesaving treatments.
Moreover, the landscape for finding clinical trials has evolved significantly. Platforms like EmergingMed have emerged, simplifying the process for patients to locate trials tailored to their condition, stage, and location. Such services exemplify the progress from the once daunting search on the National Institute of Health’s website to more user-friendly, streamlined tools.
However, patients must be aware of the costs involved, including travel, testing, and possible income loss, even if the treatment in the trial itself incurs no extra charge. Some sponsors may cover these additional expenses, alleviating the financial burden on participants.
The ethical considerations surrounding participant compensation in clinical trials have also shifted. It is increasingly recognized that it is fair to reimburse participants for their contributions, including expenses and the opportunity costs associated with their involvement. This aligns with the broader societal recognition of compensating those who contribute to public welfare, such as emergency responders.
The influence of patient organizations in the clinical trial domain has grown, with over 3,327 organizations in the U.S. alone, and about 18% focusing on rare diseases. These organizations have become crucial in disseminating information and raising awareness about diseases and potential treatments, with many formed in the last decade. Collaborations between patient organizations and life sciences companies have led to nearly 700 deals worth an estimated $2.4 billion in 15 years, demonstrating the power of these partnerships in advancing healthcare goals.
CROs like CMIC not only offer tailored solutions but also represent the collaborative spirit essential in today's complex healthcare challenges. The collective strengths of CROss, patient organizations, and other stakeholders are invaluable in driving the progress of medical research and ultimately enhancing patient outcomes.
Role of CROs in Medical Research
Clinical research organizations (CROs) are integral to the advancement of medical science, bringing together diverse expertise to streamline the progression from experimental compounds to approved treatments. Their role extends beyond mere regulatory compliance and logistical management; they are at the forefront of employing innovative methodologies and fostering collaborative environments crucial for complex research fields like CNS/neurology. By harnessing the power of large language models trained on voluminous clinical trial protocols, CROss like Lindus Health are redefining how research documents are crafted, enhancing clarity and accessibility for all stakeholders involved.
The importance of multidisciplinary input becomes evident when considering the challenges faced in patient recruitment—often a trial's Achilles' heel. Trials with stringent inclusion criteria, while scientifically robust, can falter without the practical considerations of patient lifestyles and treatment norms. CROss address these challenges by ensuring the operational feasibility of trials and incorporating the invaluable perspectives of clinical operations specialists and patients themselves.
Furthermore, the real-time monitoring of trial data through machine learning models exemplifies how CROs are minimizing risks and maximizing safety. This proactive approach is evident in partnerships like that between Boston IVF and CROs, where shared interests in improving success rates for complex procedures like IVF drive innovation and research.
In the evolving landscape of healthcare, where telehealth companies like Lemonaid Health strive for simplicity amidst market challenges, CROs are pivotal in optimizing strategies for growth and efficiency. Similarly, the scientific community's leap forward in gene editing, as reported in Science, underscores the necessity for CROs' strategic planning and execution capabilities, especially in trials targeting hard-to-reach HSCs for conditions like sickle cell disease.
At the heart of this ecosystem is the clinical research workforce—a cadre of research coordinators and professionals whose dedication and expertise are the bedrock of successful trial conduct. Despite the often underrecognized nature of their role, these coordinators manage a spectrum of responsibilities, from protocol development to biospecimens banking, essential for generating the high-quality data that propels medical advancements.
Amidst a national shortage of experienced clinical research professionals and a high turnover rate, the demand for these skilled individuals continues to climb. With a predicted 9.9% job market growth for clinical research coordinators between 2016 and 2026, the role of CROs in nurturing and sustaining a vibrant research workforce becomes all the more critical. The annual trend report from the IQVIA Institute for Human Data Science reflects the dynamic nature of biomedical research, highlighting the ongoing investment and belief in the value of research programs worldwide.
In essence, CROs are not just facilitators but innovators and collaborators, essential in the quest to turn scientific discovery into healthcare solutions. Their ability to adapt, integrate new technologies, and prioritize the human element within clinical trials is foundational to the industry's future success.
Benefits of Partnering with CROs
Contract Research Organizations (CROs) serve as pivotal partners for entities within the pharmaceutical, biotechnology, and medical device sectors. The expertise that CROss bring to clinical trial management optimizes the trajectory of new therapies and medical devices to market, making the collaboration mutually beneficial. CROss contribute to clinical trials by ensuring strict adherence to regulatory guidelines, which is an intricate process requiring nuanced understanding.
Their expansive networks can significantly expedite patient recruitment, a critical phase that can otherwise delay the trial process. Utilizing the established infrastructure of a CRO can lead to efficiencies that not only accelerate the development cycle but can also translate to cost savings for sponsors. Moreover, the alignment of objectives between CROss and their partners ensures that the overarching goal of advancing patient care through innovative treatments is met, underscoring the shared commitment to improving health outcomes.
Types of Services Provided by CROs
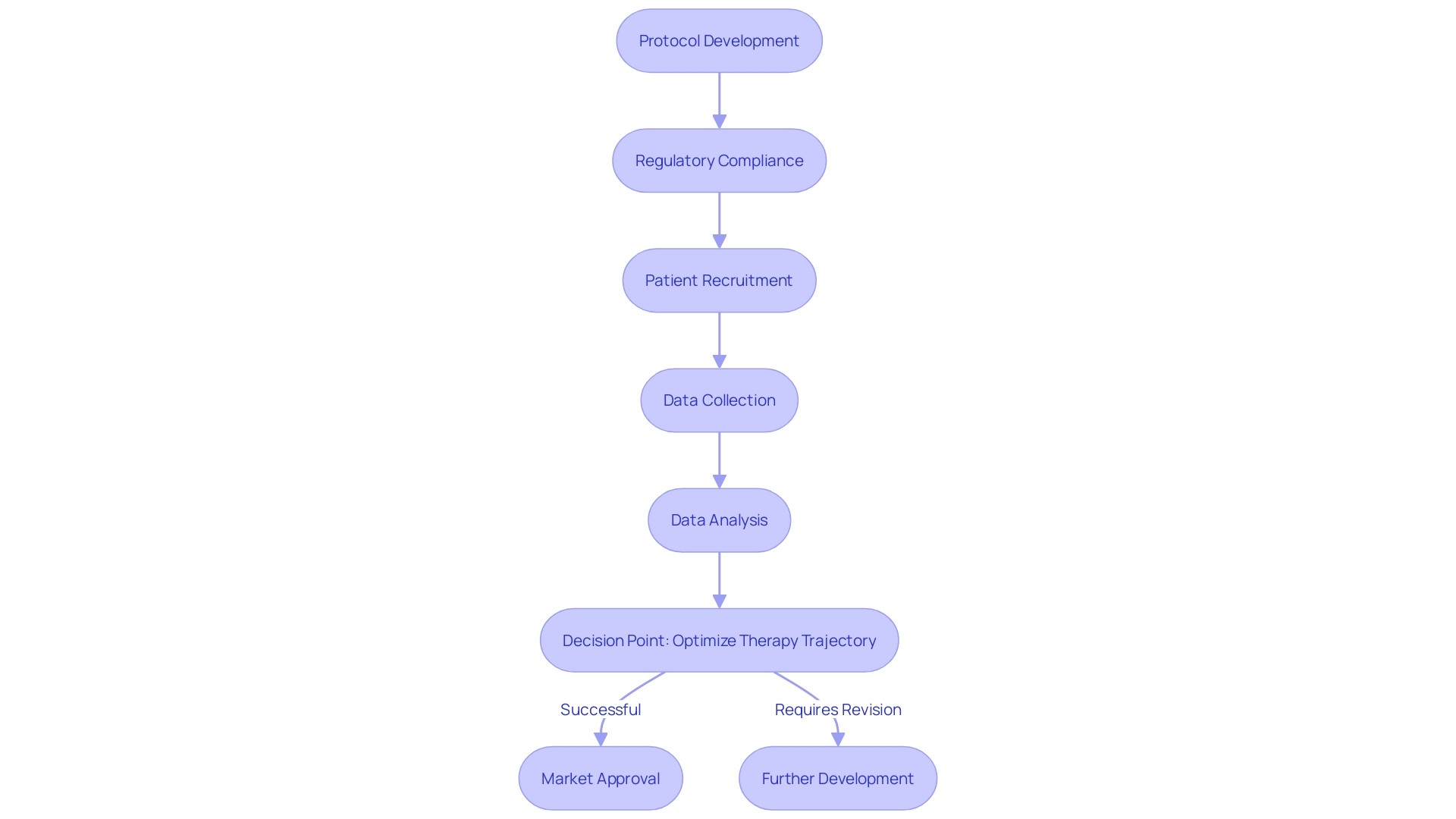
Contract Research Organizations (CROs), such as CMIC Group – Japan's pioneering CRO, offer a comprehensive suite of services that cater to every phase of drug development. Their expertise spans a broad spectrum, from protocol creation to site management, and from patient recruitment to data analysis. Their services are infused with advanced technological tools and methodologies, and they bring in-depth knowledge of specific therapeutic areas to the table.
CROs like CMIC have the capability to customize their offerings to align with the unique demands of each clinical trial. This ensures that trials are not only conducted with precision and adherence to regulatory standards but are also executed efficiently. For instance, CMIC Group extends its services to cover the entire pharmaceutical value chain, which includes contract development, manufacturing, and even market entry solutions, thereby delivering end-to-end support for their clients.
Moreover, the role of CROs is expanding, as evidenced by the recent findings from Advarra's 2023 Study Activation Survey. It highlights that nearly 60% of clinical research sites have seen an uptick in study volume, and with more sponsor-provided technology to manage, the need for CROss to offer streamlined and integrated services is becoming increasingly vital.
The invaluable contributions made by individuals in the field, like Chris with 13 years of experience in the medical device space and those working on multi-site studies to ensure successful research outcomes, underscore the dynamic and collaborative nature of the CRO industry. They strive not just to meet the scientific needs of a trial but also to provide a compassionate and positive experience for participants – a testament to the human-centric approach of CROs.
In the face of a latent workforce crisis, CROs are also poised to play a crucial role in reshaping the clinical research landscape. They are actively engaging in the conversation about defining professional identities within the clinical research workforce, as highlighted in the report by the National Academies of Science, Engineering, and Medicine.
As clinical trials remain the cornerstone for advancing medical treatments, the collaboration between CROs, pharmaceutical companies, medical device manufacturers, and research institutions is more important than ever. Through their comprehensive services and dedication to both science and patient welfare, CROs are instrumental in driving healthcare innovation forward.
Case Studies: Successful Medical Research Projects with CROs
Clinical research organizations (CROs) have become pivotal partners in the realm of medical research and development, particularly in the field of oncology and rare diseases. Notable for their role in expediting the clinical trial process, CROss have enabled significant achievements in medical science. One such instance is the partnership between IceCure Medical and Terumo Corporation, which has been instrumental in advancing the cryoablation technology for breast cancer treatment.
This collaboration is poised to make strides in the Japanese market, with regulatory approval anticipated in the near future. The involvement of CROss in such initiatives underlines their capacity to manage complex trials, facilitate rapid patient recruitment, and ensure high-fidelity data collection, ultimately contributing to the approval of new, lifesaving treatments.
The impact of CROs extends beyond the traditional clinical trial framework. In the telehealth sector, companies like Lemonaid Health are confronting the challenges of growth and market penetration by leveraging CRO expertise to streamline their media strategies. This approach demonstrates the adaptability of CROss in addressing the unique needs of diverse health services, including telehealth, where customer acquisition and patient engagement are critical.
Furthermore, the significance of CROs is highlighted by their support in the investigation of rare diseases. The story of Dr. Alexander Marneros's spontaneous research into aplasia cutis is a testament to how CROs can assist in uncovering the genetic underpinnings of rare conditions, thereby opening pathways to targeted therapies and a deeper understanding of these diseases.
The relationship between CROs and patient organizations also underscores the CEO's role in enhancing patient-centric care. With numerous patient organizations partnering with life sciences companies, CROss have become integral in facilitating these collaborations, which focus on educating patients, advancing treatment options, and emphasizing the importance of the patient experience in medical research.
In conclusion, the value of CROs in driving successful medical research projects is indisputable. Through strategic partnerships, they bring together the expertise needed to navigate the challenges of today's diverse healthcare landscape, from addressing the needs of individual patients to accelerating the arrival of novel treatments to the market.
Challenges and Considerations in Working with CROs
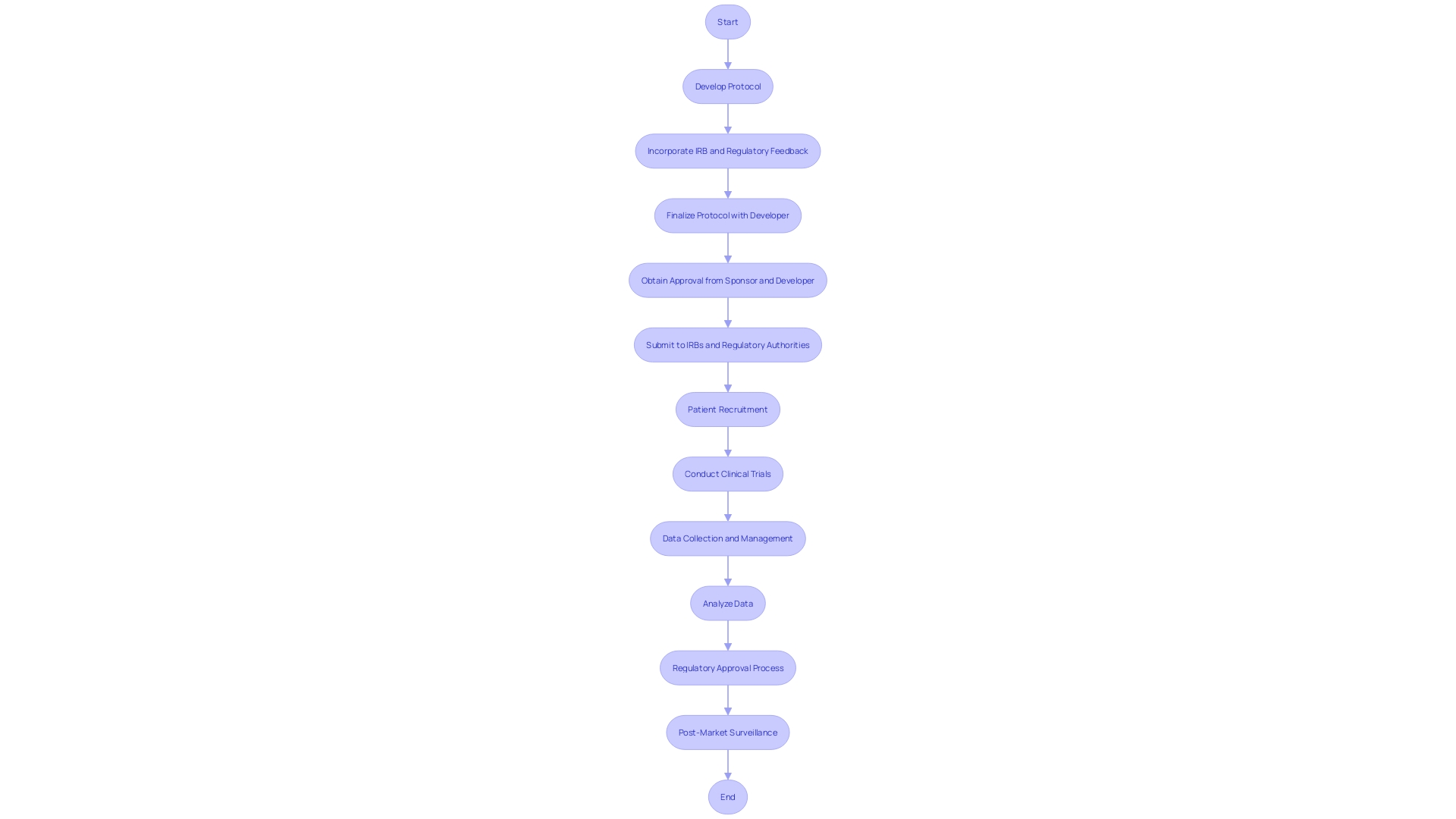
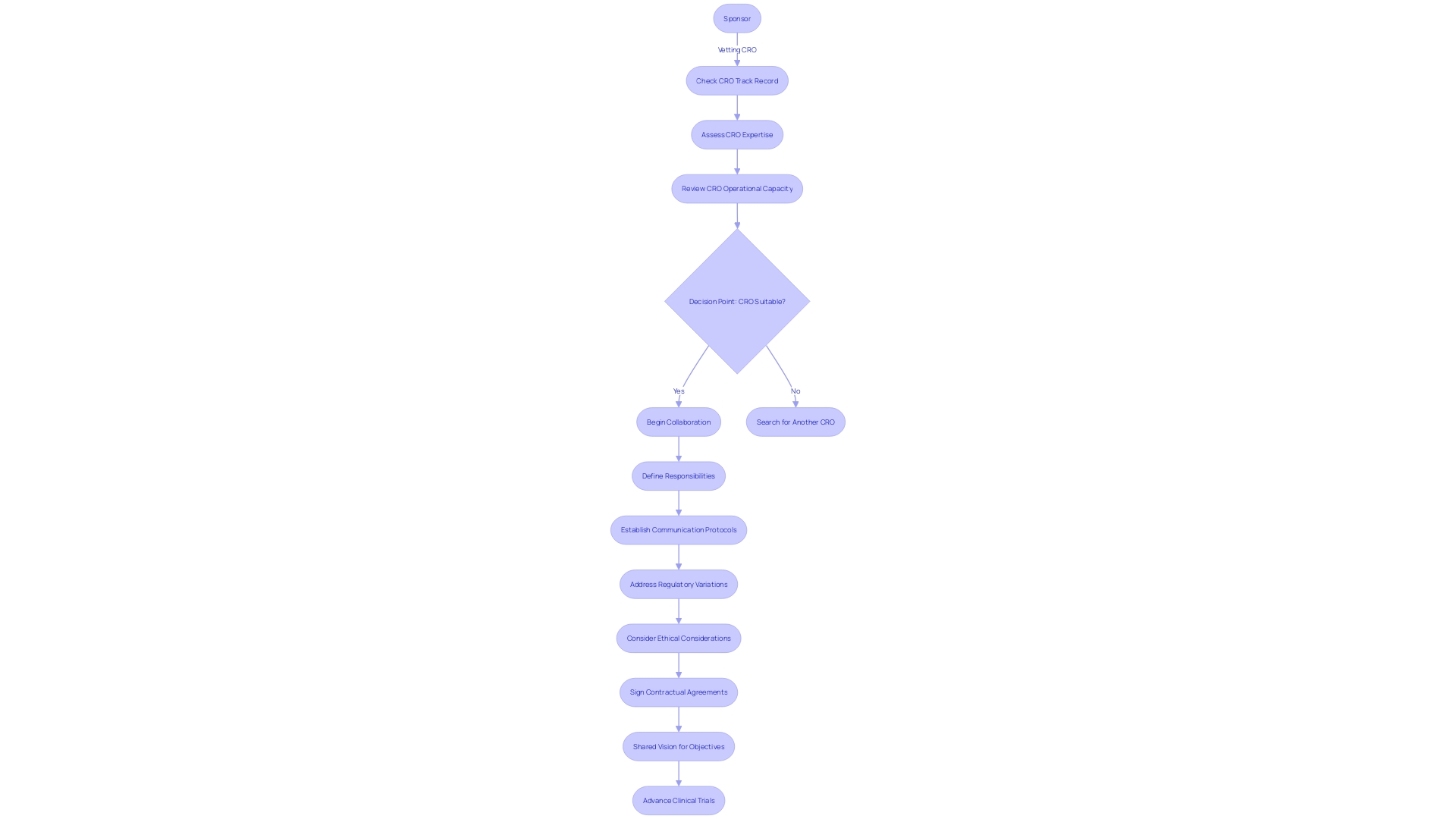
Securing the collaboration of a Clinical Research Organization (CRO) can be instrumental in advancing clinical trials, but it necessitates a meticulously planned approach to communication, roles, and contractual terms. The crux lies in ensuring that the sponsor and the CRO are in lockstep throughout the trial process. This harmony is achieved through well-established communication protocols, clearly defined responsibilities, and a shared vision for the project's objectives.
Moreover, vetting a CRO's track record, expertise, and operational capacity is a pivotal step in selecting a partner that aligns with the sponsor's expectations.
Case studies, such as the use of Electronic Health Record (EHR) data in multi-center pharmaceutical trials, demonstrate the need for a central coordinating center to navigate the technical and governance challenges associated with integrating digital data into trial operations. This highlights the importance of a CRO's ability to manage complex trial components effectively.
Furthermore, the evolving landscape of clinical trial regulations, such as the differing definitions of non-interventional studies by the FDA and EMA, underscores the necessity for sponsors to establish agreements with CROs that are adaptable to international regulatory variations. By anticipating and addressing these challenges in the contractual phase, sponsors can avert potential complications that may arise due to regulatory discordance.
In this dynamic environment, the ethical considerations of participant compensation also come to the fore. As stakeholders in the clinical trial ecosystem emphasize, it is unjust to overlook the financial burdens borne by trial participants. Fair treatment, including appropriate reimbursement for expenses and risks, is an ethical imperative that should be reflected in the design and execution of clinical trials, and by extension, the agreements with CROs.
Ultimately, the goal is to strike a balance between leveraging the benefits of CRO partnerships and navigating the inherent complexities with foresight and due diligence. This includes considering the rapid growth of decentralized clinical trials, expected to surge at a compound annual growth rate of 30.1% from 2021 to 2026, and the accompanying need for standardization, ethical clarity, and data security. By adhering to these principles, sponsors can ensure the integrity and success of their clinical trials.
Future Trends in CROs and Medical Research
As the landscape of clinical research shifts, Contract Research Organizations (CROs) are at the forefront of embracing innovative methodologies that address the evolving needs of the medical field. The global decentralized clinical trial market, a burgeoning sector, is anticipated to surge at a compound annual growth rate of 30.1% between 2021 and 2026. Decentralized Clinical Trials (DCTs) are transcending geographical barriers, enabling patients, such as those in rural Pennsylvania battling rare diseases, to participate in clinical studies without the need for extensive travel, which aligns with the principle that trials should reach patients 'at home', thereby addressing clinical trial deserts.
The integration of real-world data is another transformative trend, enhancing the contextual relevance of clinical trials and shaping a more comprehensive understanding of therapeutic outcomes in everyday healthcare settings. This is coupled with the increasing utilization of digital therapeutics—software-driven treatments deployed via mobile devices, virtual reality, and sensors—although their integration into clinical trials is still in its infancy, partly due to limited insurance coverage.
Moreover, the potential of Artificial Intelligence (AI) and Machine Learning (ML) in revolutionizing clinical trials is drawing attention. These technologies promise to refine data analysis, personalize treatments, and potentially lead to the conception of self-driving clinical trials. As noted by industry leaders, AI and ML will play a crucial role in expediting drug discovery and development.
However, the introduction of these technologies is not without challenges. Concerns around standardization, regulatory and ethical uncertainties, data security, and equitable technology access must be addressed. As we look to the future, the successful implementation of these trends will rely on a commitment to developing best practices that ensure the quality and integrity of clinical trials, a sentiment echoed by thought leaders and key industry reports.
Call to Action
Pharmaceutical, biotechnology, and medical device companies continuously seek ways to expedite the development of innovative therapies and enhance patient outcomes. Partnering with a Contract Research Organization (CRO) offers a strategic advantage, providing access to specialized expertise, extensive resources, and a broad network. The trust engendered through successful collaborations, as evidenced by GoodRx's high net promoter score and significant user base, underscores the value of reliable partnerships in medical research.
In addition, collaborations with CROs that employ cutting-edge technologies like machine learning models for real-time trial monitoring can further reduce errors and improve safety outcomes.
Lindus Health's approach to CNS/neurology research, which prioritizes multi-disciplinary collaboration, highlights the importance of incorporating diverse perspectives, including patient voices, to ensure the feasibility and success of clinical trials. Similarly, Lemonaid Health's telehealth services demonstrate the critical role of innovation in addressing complex challenges in healthcare delivery and patient engagement.
The integration of artificial intelligence in drug development, as practiced by Genentech, exemplifies the transformative potential of technology when combined with human expertise. With the medical device sector rapidly evolving, publications such as Medical Device News Magazine serve as valuable resources for professionals and patients alike, indicating a strong interest in the latest developments and technological advancements in healthcare.
Controlled medical research studies, which are foundational to the advancement of medical knowledge, must be meticulously designed to address well-defined research questions and minimize biases. Whether it's addressing the challenges faced by patients with rare diseases or ensuring laboratory safety, the collective efforts in medical research are geared towards improving health outcomes and enhancing the quality of life for patients worldwide. By defining a shared purpose and investing in infrastructure that supports research and innovation, the medical community can continue to make strides in the discovery and implementation of new diagnostics, treatments, and preventative strategies.
Conclusion
In conclusion, Clinical Research Organizations (CROs) are instrumental in advancing medical research and enhancing patient outcomes. They offer a wide range of services that address logistical challenges, facilitate cross-border collaborations, and simplify the process of finding tailored clinical trials. CROs contribute to ethical considerations and collaborate with patient organizations to raise awareness about diseases and potential treatments.
CROs play a critical role in driving medical research progress by bringing together diverse expertise and employing innovative methodologies. They ensure operational feasibility, incorporate valuable perspectives in patient recruitment, and minimize risks through real-time data monitoring. CROs also optimize strategies for growth and efficiency in the evolving healthcare landscape.
Partnering with CROs benefits pharmaceutical, biotechnology, and medical device companies by ensuring adherence to regulatory guidelines, expediting patient recruitment, and translating to cost savings. The alignment of objectives between CROs and partners underscores the commitment to advancing patient care through innovative treatments.
CROs offer comprehensive services that cater to every phase of drug development. They customize their offerings, ensuring precision, regulatory compliance, and efficiency in clinical trials. Successful projects demonstrate CROs' ability to manage complex trials, facilitate rapid patient recruitment, and ensure high-fidelity data collection.
Working with CROs requires meticulous planning, communication, and addressing challenges such as evolving regulations and ethical considerations. In the future, CROs will embrace innovative methodologies, including decentralized trials, real-world data integration, and artificial intelligence. Collaborations with CROs provide a strategic advantage, offering specialized expertise, extensive resources, and a broad network.
Controlled medical research studies, supported by infrastructure fostering research and innovation, are crucial for advancing medical knowledge and improving patient outcomes. The collective efforts of the medical community aim to enhance the quality of life through the discovery and implementation of new diagnostics, treatments, and preventative strategies. CROs play a vital role in this pursuit, driving healthcare innovation forward.




