Introduction
Conducting clinical trials in Latin America presents a unique set of challenges and opportunities, particularly in regions where healthcare infrastructure is underdeveloped. The disparities in healthcare quality and accessibility significantly impact the feasibility and success of clinical research. Cultural differences, logistical hurdles, and regulatory complexities further complicate the recruitment and retention of participants.
However, collaborative efforts and tailored strategies are being implemented to address these issues and improve the efficacy of clinical trials. This article explores the multifaceted landscape of clinical research in Latin America, highlighting the obstacles, advantages, regulatory frameworks, and ethical considerations essential for advancing medical science in the region.
Challenges in Conducting Clinical Trials in Traditional Regions
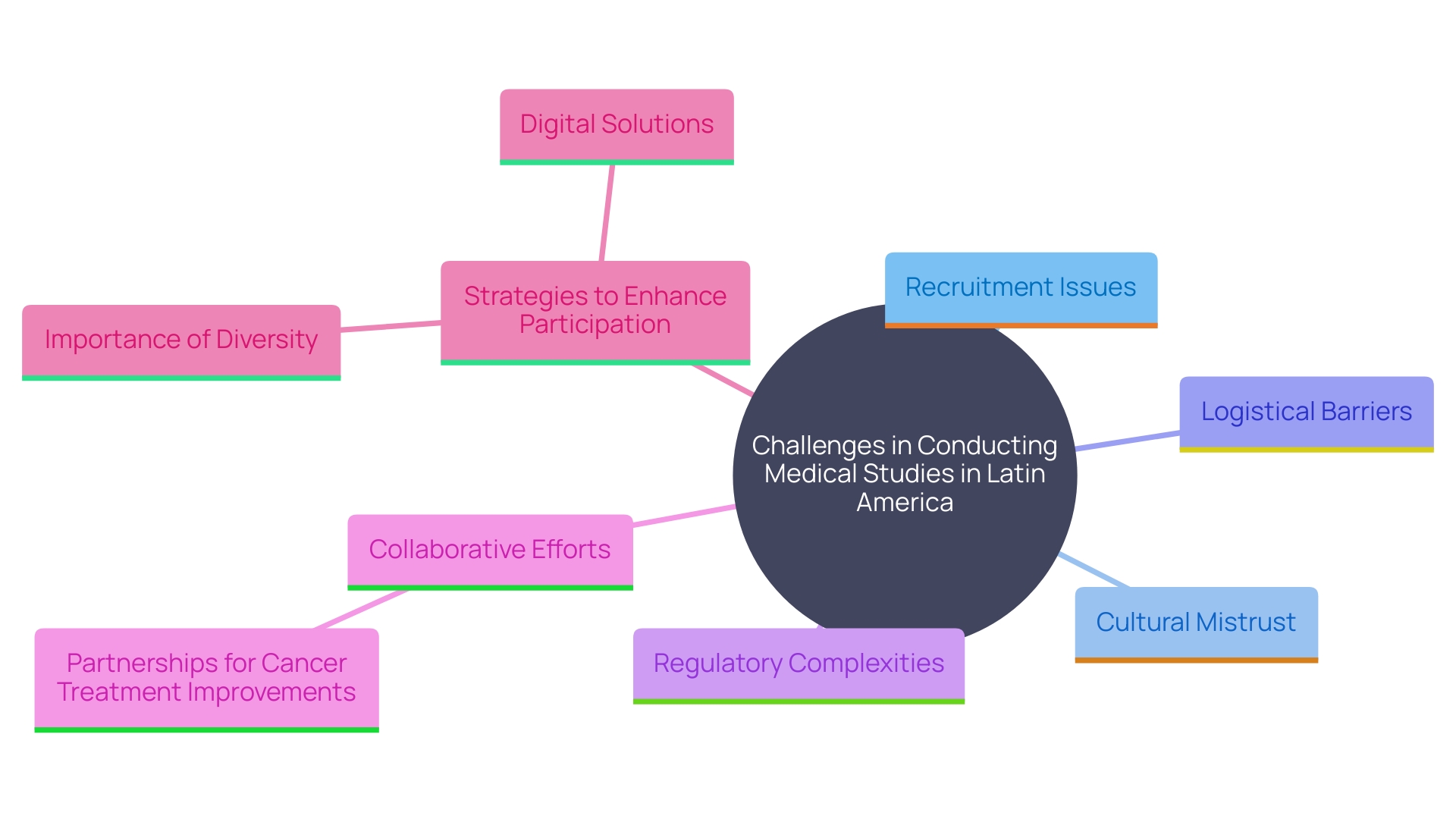
Carrying out medical studies in Latin America offers distinct obstacles, especially in areas where healthcare systems are lacking. For instance, in many low- and middle-income countries (LMICs), it is estimated that around 8 million people die annually from treatable conditions, with 60% of these deaths due to poor-quality healthcare. This underscores the importance of robust health systems capable of delivering high-quality care across various contexts.
Recruitment challenges are often worsened by cultural differences and mistrust in research involving health. Cervical cancer, one of the most treatable forms of cancer with early detection, still claims numerous lives due to low participation in preventive screening, such as the Papanicolaou Test, even when offered for free. This is a critical issue, particularly in developing countries, where preventive health behaviors are not widespread.
Moreover, logistical challenges such as transportation and communication barriers further complicate the recruitment and retention of participants. Sophisticated medical technologies and steady oversight procedures are frequently insufficient, which can postpone or obstruct research trials. For example, the variability in regulatory frameworks across different Latin American countries adds another layer of complexity.
However, collaborative efforts are underway to address these challenges. For instance, the opening of new cancer centers in Brazil, in partnership with Dana-Farber Cancer Institute, aims to elevate the standard of oncology services and ensure that Brazil remains at the forefront of cancer treatment. This collaboration includes training programs and technology transfers to enhance medical education and patient care.
To overcome these obstacles, tailored strategies must be implemented. Utilizing digital government capabilities, as observed in Montevideo, Uruguay, where health information was consolidated across different systems, can enhance patient monitoring and increase efficiency in studies. Furthermore, encouraging diversity in medical research is essential. Studies have shown that diverse teams are more productive and creative, leading to better science and more inclusive health outcomes. This method guarantees that the advantages of research studies are fairly shared, enhancing overall patient enrollment and involvement in South America.
Advantages of Conducting Medical Device Trials in Latin America
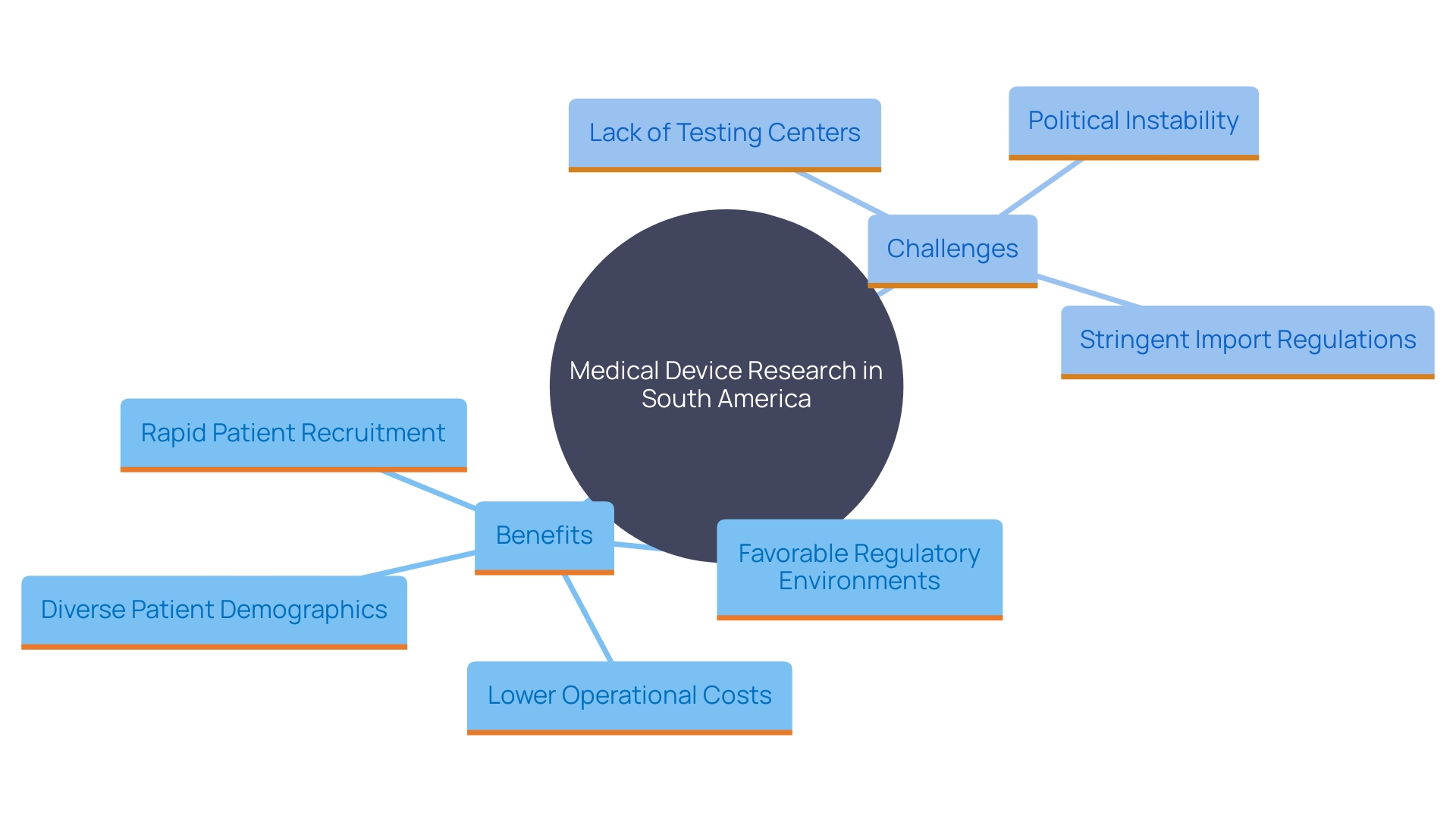
The region of America offers significant benefits for carrying out medical device research, featuring a varied patient demographic that improves the applicability of study findings. The regulatory environment in the region is becoming increasingly favorable, as many countries streamline approval processes for research trials. Additionally, the operational costs are significantly lower compared to North America and Europe, making Latin America an attractive option for sponsors. The ability to recruit patients more rapidly is another key benefit, particularly due to the high prevalence of certain diseases in the region.
Furthermore, the integration of real-world data into medical research is gaining traction, providing a more precise overview of the expenses and efficacy of healthcare choices. At the recent Outsourcing in Clinical Trials: Medical Devices Europe 2024 conference, experts discussed the potential of artificial intelligence and real-world data to enhance clinical studies. These advancements can enhance regulatory submissions and improve study efficiency.
However, it's essential to acknowledge the challenges. Nations in South America may lack established testing centers and suitable research support personnel, necessitating education on testing practices and guidelines. Political instability and stringent import regulations can also affect product availability, as observed during conflicts like the Russia-Ukraine war, where multiple clinical studies were abandoned.
In spite of these obstacles, the benefits of carrying out experiments in South America are considerable. The area provides elevated hiring rates, improved access, and a more involved investigator research team, as stated by Carine Cochereau, vice president of international oversight at Integra Life Sciences. This makes the area of South America a promising region for medical equipment tests, contributing to more effective and adaptive health policies and ultimately enhancing the quality of care provided.
Regulatory Frameworks in Latin America
Steering through the legal structures overseeing medical studies in Latin America necessitates a profound comprehension of the varied and complex guidelines that differ from nation to nation. Each nation has its own governing body that sets forth specific requirements for ethical approvals, informed consent, and reporting procedures. This complexity highlights the importance of comprehensive regulatory understanding for the successful implementation of research studies.
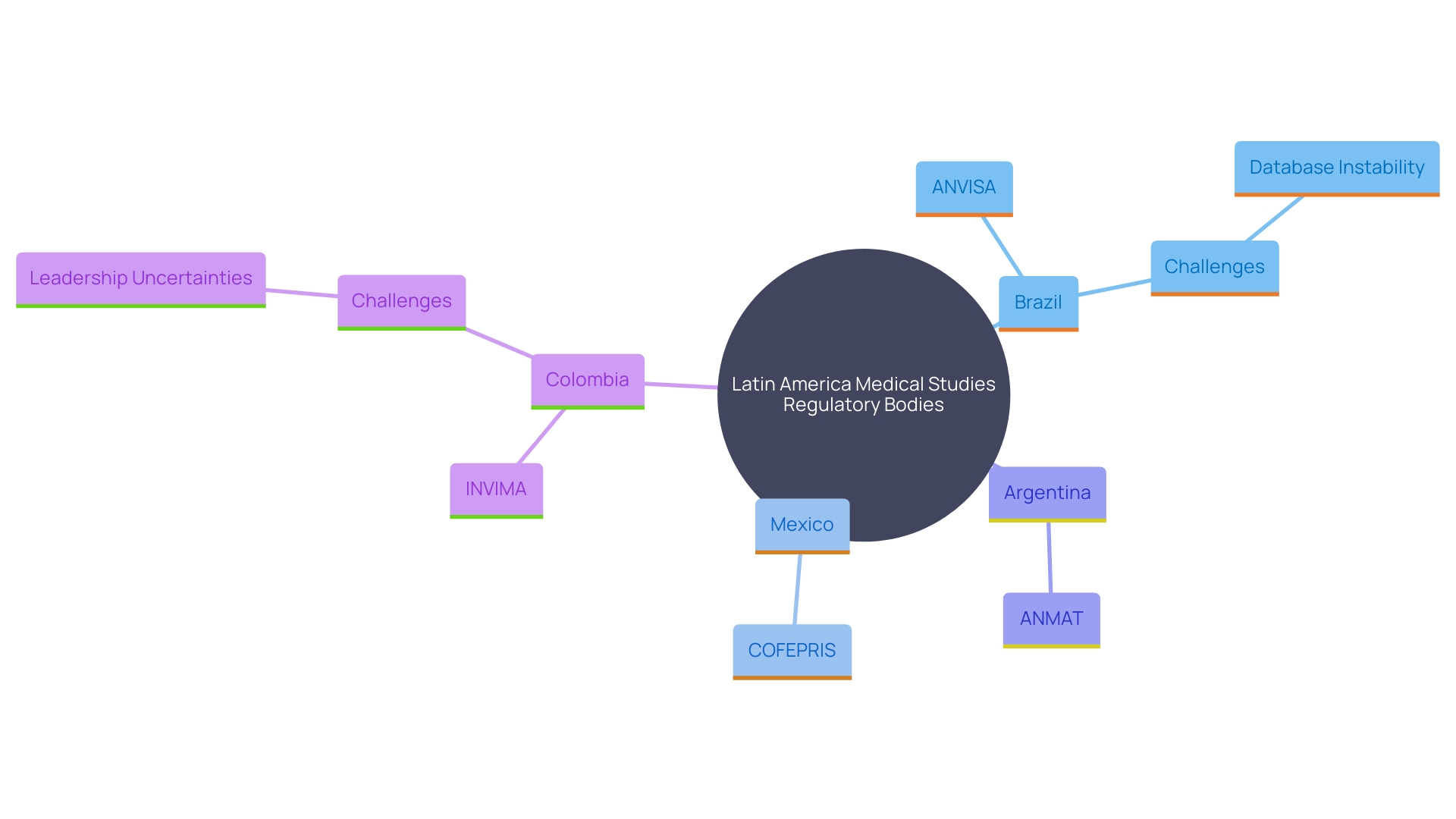
In recent years, countries such as Brazil, Mexico, and Argentina have taken significant steps towards aligning their regulations with international standards. This harmonization effort not only facilitates smoother processes but also enhances compliance, ensuring that these activities meet global benchmarks for ethical and scientific rigor.
For example, Brazil's National Health Surveillance Agency (ANVISA) plays a vital role in supervising medical studies and ensuring they comply with strict safety and ethical standards. Similarly, Mexico's Federal Commission for the Protection against Sanitary Risk (COFEPRIS) and Argentina's National Administration of Drugs, Food, and Medical Technology (ANMAT) have made considerable progress in simplifying their procedures, making it easier for researchers to navigate the approval processes.
Despite these advancements, challenges remain. For instance, the recent instability in Brazil’s regulated substances database, which has been offline since 2021, underscores the ongoing problems that can affect research operations. Furthermore, Colombia's National Institute of Drug and Food Surveillance (INVIMA) has encountered leadership uncertainties, which can lead to delays and influence the oversight environment.
Overall, the changing regulatory environment in South America offers both possibilities and difficulties for medical research execution. By remaining knowledgeable and adjusting to these changes, researchers can better oversee their studies and contribute to the progress of medical understanding in the region.
Ethical Considerations and Patient Access
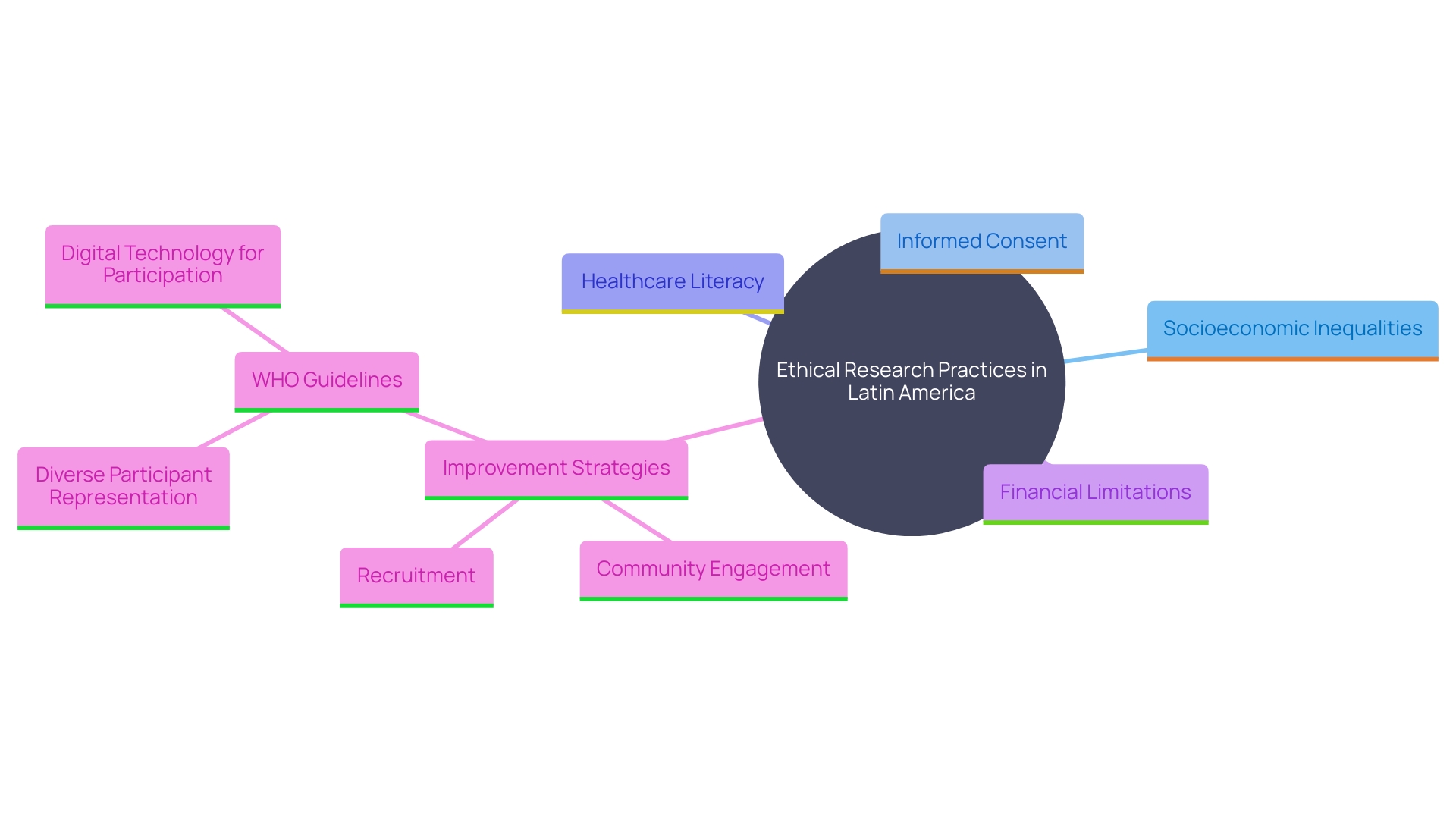
Moral factors are essential in research studies, particularly in Latin America, where socioeconomic inequalities can greatly affect patient access to study opportunities. Ensuring recruitment strategies are both ethical and inclusive is essential for protecting participants' rights. Researchers must diligently address informed consent, ensuring that patients fully comprehend the implications of their participation. Furthermore, tackling obstacles like healthcare literacy and financial limitations is essential for improving recruitment and guaranteeing fair involvement in studies.
Based on recent WHO guidelines, national health agencies should concentrate on establishing a supportive setting for research studies, which involves tackling infrastructural shortcomings and guaranteeing diverse participant representation. In 2022, there were stark contrasts in clinical study participation, with 27,133 studies in high-income nations compared to 24,791 in low- and middle-income nations. This disparity underscores the need for stronger country-led research and development ecosystems to advance health science and make interventions more accessible globally.
Involving patients and community leaders in advisory boards can offer valuable perspectives on obstacles to enrollment and improve patient-centered communications regarding the study. Interim analyses to monitor participant representativeness should be included in study protocols to avoid selection bias. The use of digital technology and social media platforms, although still underutilized, can play a significant role in reaching underrepresented groups and improving trial participation.
Recent updates to the Declaration of Helsinki emphasize the importance of meaningful engagement with participants, recognizing them as partners in research. This shift towards responsible inclusion aims to address historical research misconduct and marginalization in certain ethnic groups. By fostering community engagement and prioritizing policy innovation, we can transform clinical research in Latin America and ensure that cost is not a barrier to accessing cutting-edge medical treatments.
Conclusion
Conducting clinical trials in Latin America involves navigating a landscape marked by both challenges and opportunities. Key hurdles include underdeveloped healthcare infrastructure and cultural mistrust, which can impede participant recruitment. However, initiatives like new cancer centers and digital health integration are enhancing engagement and trial efficiency, highlighting the importance of tailored strategies.
The region offers significant advantages for medical device trials, such as a diverse patient population and a favorable regulatory environment, which can lead to lower operational costs and quicker recruitment. Nonetheless, challenges like political instability and the need for trained research staff must be addressed to maximize these benefits.
Understanding the complex regulatory frameworks is essential for successful trial execution. Recent harmonization efforts have improved compliance, although issues like database instabilities still pose challenges.
Ethical considerations are critical for ensuring equitable access to clinical trials, especially given socioeconomic disparities. Engaging local communities and utilizing technology can help overcome barriers to participation. By prioritizing ethical recruitment and fostering inclusivity, clinical trials in Latin America can significantly advance medical research and enhance healthcare outcomes.
The region is well-positioned to contribute meaningfully to global medical science, provided that its challenges are met with strategic and collaborative solutions.




