Introduction
Human Factors and Usability Engineering (HF&UE) play a vital role in the design and development of medical devices, focusing on enhancing user experience and patient safety. By integrating HF&UE principles, designers can create medical devices that are not only functional but also intuitive, reducing the risk of user error and improving healthcare delivery. This article explores the importance of human factors in medical device design, key steps in applying HF&UE, regulatory compliance, mitigating user errors through design, creating a human factors plan, and best practices for integrating HF&UE into medical device development.
It emphasizes the need for comprehensive planning, collaboration, and user-centered design to ensure the safety, effectiveness, and adaptability of medical devices in the ever-changing landscape of healthcare needs and technological advancements.
Understanding Human Factors and Usability Engineering
Human Factors and Usability Engineering (HF&UE) are essential in the creation and advancement of healthcare instruments, playing a crucial part in improving the experience of individuals and ensuring their well-being. These fields concentrate on creating designs that prioritize the needs of individuals and patients, guaranteeing that tools are tailored to effectively serve them. By incorporating HF&UE principles, designers can develop technologies that are not just efficient but also user-friendly, minimizing the chance of user mistakes and enhancing healthcare delivery in general.
The development process incorporates components, activities, and results that collectively ensure healthcare instruments fulfill their intended specifications. 'Efficient planning for creation is crucial, as emphasized in the seven vital aspects of the construction and advancement regulation.'. This meticulous planning aids in preventing the common delays seen in clinical timelines and helps in adhering to stricter compliance standards.
Automation and AI are increasingly playing a role in this field, with AI being seen as an extension of automation—adding complexity to inputs and outputs, which is particularly relevant when interpreting textual data. Efficient adherence is crucial in development of healthcare equipment, particularly after release when enhancements may be required due to changing user expectations or modifications in interconnected systems.
Custom healthcare software solutions have become a transformative force, aiming to enhance patient care and streamline operations. The development of such solutions requires a deep understanding of unique organizational requirements, underscoring the importance of HF&UE in crafting patient-centric and efficient healthcare tools. Furthermore, as the FDA concentrates on developing home prototypes to test healthcare equipment in different living conditions, it becomes evident that the design and user-friendliness of these instruments must be taken into account in diverse environments.
The industry is also witnessing a digital revolution in quality management, with the adoption of digital solutions and AI innovations that are reshaping healthcare. The FDA's recent guidance on incorporating cybersecurity into the quality system management highlights the importance of secure software practices throughout the Total Product Life Cycle, ensuring the safety and effectiveness of healthcare equipment amidst cyber threats.
To recap, Human Factors and Usability Engineering are not only focused on meeting standards; they are concerned with developing instruments that are secure, efficient, and capable of adjusting to the evolving healthcare requirements and technological progress.
Importance of Human Factors in Medical Device Design
Recognizing the significance of human factors in the development of medical instruments is vital for improving usability, efficiency, and safety. The development procedure should consider the extensive requirements of all individuals, encompassing patients, healthcare experts, and technicians responsible for equipment upkeep. Historical examples, such as the flawed design of the Dalkon Shield IUD, demonstrate the dire consequences of disregarding human factors, where designers overlooked critical aspects like cervical pain, infection risk, and removal mechanics. Moreover, contemporary healthcare obstacles highlight the requirement for tools that take into account the comprehensive user experience, encompassing emotional, physical, and practical considerations. This approach is highlighted by research on patient-clinician dynamics, where factors such as treatment accessibility and cost are as pivotal as clinical outcomes. Furthermore, progressions in healthcare technology, like tailored software that complies with HIPAA and improves patient care, additionally demonstrate the increasing convergence of human factors engineering and advanced equipment development. Adopting this mindset not only reduces risks but also places products at the forefront of market innovation, creating tools that are not only safe but also enjoyable to use, in line with regulatory standards, and provide a competitive advantage.
Key Steps in Applying Human Factors and Usability Engineering
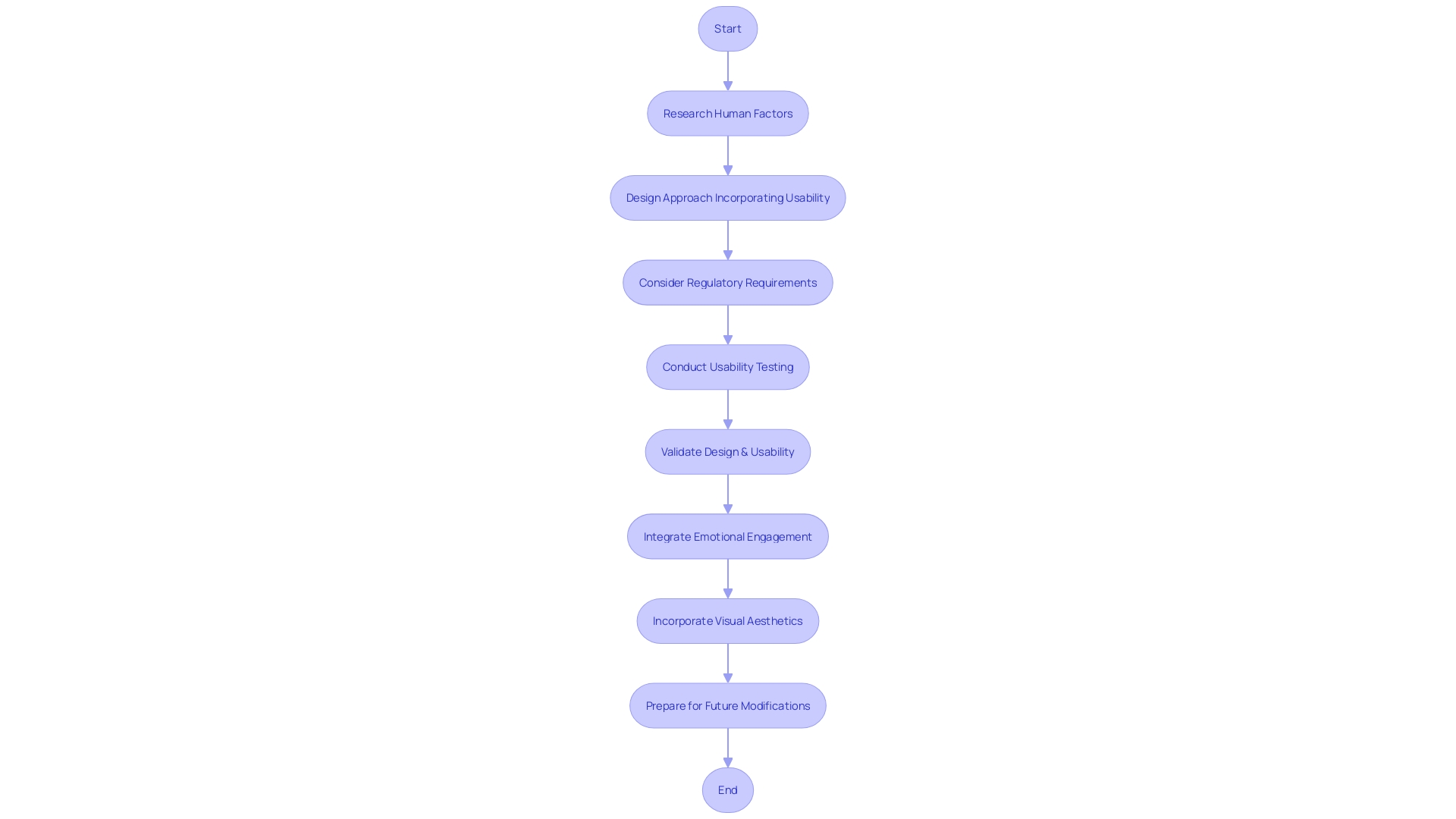
The incorporation of human factors and usability engineering into the design of medical instruments is a multi-step procedure that guarantees the final outcome satisfactorily fulfills the requirements of its users. At the beginning, thorough research with individuals must be conducted to gather insight into the needs and contexts of use, which can include clinicians, patients, and maintenance personnel. Incorporating feedback from these stakeholders is crucial, as real-world experiences can significantly influence the device's development path. As an example, Morven Shearlaw emphasizes the significance of honest clinician feedback, which can shift a product's development to better meet the needs of its intended users.
After the research phase, a design approach focused on the individual is adopted, which includes iterative design and prototyping, allowing for refinements based on user interaction. This approach aligns with the principles of Agile methodology and application lifecycle management (ALM), supporting ongoing updates and improvements to the product. Regulatory considerations are also paramount, as Morven Shearlaw emphasizes, "Quality management is about quality, it's not just about proving that you can sell in the market because you've got a certain certification."
Usability testing and validation are then conducted to confirm that the product meets the intended goals and provides a positive experience for the individuals. This involves methods such as diary studies, where users record their interactions with the object over time, providing valuable data for assessing the object's performance in real-world settings.
As the healthcare equipment sector progresses, the significance of emotional engagement and visual aesthetics becomes more and more acknowledged. Medical blogs discuss trends and insights that remain relevant, such as the significance of labels for traceability and the need for clear differentiation between marks like the CE mark and the China Export mark.
Ultimately, in this period of healthcare transformation, where technology plays a crucial role, the creation and advancement of healthcare tools must be versatile to adjust to future modifications. The opening of MedStar Georgetown's Verstandig Pavilion, as described by Lisa Boyle, MD, is a testament to this, merging compassionate care with cutting-edge technology to meet healthcare needs for decades to come.
Custom healthcare software solutions, as opposed to off-the-shelf applications, offer tailored, patient-centric, and secure options that address the unique requirements of healthcare providers, ultimately enhancing patient care and streamlining operations. This method for developing and creating healthcare technology is both a reaction to and an influencer of the ongoing progress in the industry.
Regulatory Compliance and Standards
Ensuring that healthcare equipment is designed in compliance with the necessary regulatory standards is an indispensable aspect of product development. Current guidelines from organizations such as the FDA and the international standard IEC 62366 are crucial for incorporating human factors and usability engineering into the design process of healthcare instruments. With a focus on quality assurance and a systematic approach to meeting quality standards, regulatory compliance must be woven into every stage of development, from initial concept to post-market surveillance. The crucial importance of these standards is emphasized by the potential risks of non-compliance, which can vary from regulatory sanctions to significant impacts on patient safety and equipment efficacy.
Advances in healthcare technology, like the use of augmented and virtual reality in home health assessments by the FDA, underscore the importance of adaptability and resilience in the development process. This innovative approach reflects the regulatory bodies' commitment to understanding how medical instruments operate in diverse environments, ensuring they meet users' needs effectively. It's imperative for manufacturers to consider these evolving methods and incorporate the feedback from stakeholders, such as patients and healthcare providers, to align with these standards.
The FDA emphasizes patient safety as its top priority, necessitating that devices not only meet specifications and manufacturing specifications but also prioritize patient outcomes. In this undertaking, the FDA's expectations for In Vitro Diagnostics (IVDs) demonstrate the significance of accurate and reliable results, which depend on well-defined controls and comprehensive clinical data. Furthermore, the FDA's Center for Drug Evaluation and Research (CDER) offers guidance to developers on study structures and data prerequisites, reflecting the agency's dependence on evidence-based decision-making.
Quality assurance is a multi-faceted operation, with design control ensuring that products meet predefined requirements and address potential risks, and process validation confirming manufacturing consistency. The modification of regulatory documents, relying on real-life implementation and case studies, provides updated guidance on drug-apparatus combinations, co-packaged products, and companion diagnostics, all of which are necessary for ensuring compliance with the recently updated EU regulations for healthcare instruments.
In brief, the convergence of regulatory adherence, quality assurance, and human factors engineering is crucial to delivering products that are both safe and effective. By prioritizing these elements throughout the creation and development process, manufacturers can better navigate the regulatory landscape and contribute to advancements in patient care.
Mitigating User Errors through Design
Innovative principles are vital when developing devices to minimize user errors and enhance patient safety. Error-proof design, an approach grounded in the theoretical framework discussed in the paper Designing for Complementarity, aims to facilitate complementarity in decision-making processes within healthcare. This methodology incorporates features such as Feature Importance, Counterexample Explanations, and Similar-Case Explanations, which can significantly bolster the error-proof capabilities of AI-based tools.
Security in the field of medicine is also an important aspect, as highlighted by the FDA's guidance on Cybersecurity in Medical Devices. Ensuring the safety and efficiency of the components within the broader system they function is crucial to avoid negative occurrences caused by online risks, which could vary from depleting battery life to enabling unauthorized control.
Furthermore, the implementation of automation in healthcare equipment, as outlined by the FDA, functions as a vital cause-and-effect mechanism. Modern AI is essentially an extension of this automation, with the added complexity of interpreting complex inputs and providing nuanced outputs. This is especially important in life-or-death circumstances in the healthcare sector, where the dependable and precise functioning of equipment can determine the survival of patients.
As digital patient communication transforms, with reminders and alerts becoming more prevalent, the role of visual cues and feedback in device interfaces becomes increasingly vital. This method, combined with extensive training and education for individuals, is essential in decreasing the occurrence of errors made by individuals.
Statistical data illustrates the intricate challenges encountered in the healthcare sector, emphasizing the necessity for ongoing evaluation and enhancement of system structures, workflow concerns, and personnel training. A study on electronic medication management systems (EMMs) underscores the necessity for ongoing evaluations to enhance medication safety and reduce errors.
To guarantee the safety and efficacy of healthcare delivery in a digital and AI-integrated environment, priority should be given to intuitive interfaces for healthcare devices, robust cybersecurity measures, and effective training for users.
Creating a Human Factors Plan for Medical Devices
The essence of human factors in the creation of healthcare equipment is to optimize both usability and safety, a process that requires meticulous planning and collaboration. A thorough plan on human factors involves identifying important parties involved and establishing requirements of the users, which are crucial in ensuring that the equipment meets the needs of all end-users. Throughout the planning and development lifecycle, integrating human factors activities is not merely a suggestion—it's an industry mandate to prevent misuse and enhance patient care.
To craft an effective human factors plan, multidisciplinary approaches are paramount. These approaches should draw on lessons from successful health technology companies that prioritize patient- and people-centric innovation. For example, consider the synergy between entrepreneurship and technology that propelled Philips' growth. This kind of collaboration is key to identifying what customers truly need and bringing meaningful innovations to healthcare.
Current industry challenges highlight the importance of such human factors planning. The recent executive brief by a prominent healthcare expert highlights the need to address design flaws in home-use instruments, stressing the importance of taking into account the average individual during the development process to prevent any potential damage. This aligns with the systematic approach of quality assurance in MedTech, which is not just about compliance but delivering safe and effective products. Design control and process validation are critical components of this, ensuring that potential risks are mitigated and quality standards are consistently met.
With the rapid pace of technological advancements and complex global supply chains, the challenges of quality assurance in MedTech are profound. Stringent regulations by bodies like the FDA and EMA demand rigorous compliance, while market competition pressures companies to accelerate development without sacrificing quality. A notable instance from QMB's client project underscores the importance of addressing quality assurance issues promptly.
In this dynamic landscape, companies must be agile in managing upgrades post-product launch. The philosophy of simplifying adherence through quality system procedures ensures that any new healthcare apparatus remains safe and effective, meeting user needs even as it evolves.
The year 2023 has been a crucial one for the healthcare equipment industry, characterized by important events from AI regulation to substantial layoffs. As we move into 2024, it's crucial for industry professionals to stay informed and adapt to the ever-changing environment. The recap provided by Medical Device Network serves as a tool to process these developments and prepare for the future.
By comprehending these complex challenges and foreseeing the requirements of individuals, a human factors plan can exceed a compliance checklist; it can serve as a roadmap for innovation and a catalyst for advancing patient-centric technologies that transform healthcare for the better.
Best Practices for Integrating Human Factors into Medical Device Development
Incorporating human factors and usability engineering into medical equipment development is essential for producing products that not only satisfy regulatory requirements but also achieve high levels of satisfaction and effectiveness among users. Including end-users from the initial stages of development guarantees that their requirements and work settings are taken into account, resulting in user-friendly and safer products. Iterative development and usability testing are crucial, enabling developers to refine their products based on feedback from actual individuals, which is necessary for identifying potential use errors and enhancing the overall user experience.
A strategic approach to human factors integration involves starting with a clear vision of who the end-users are, the impact the tool will have, and the value it brings. This vision should guide the development process, with milestones designed to validate that the product meets its intended goals. Companies like Medtronic exemplify this by delivering innovative technologies that directly address user needs, transforming lives every second, and showcasing the importance of a user-focused design philosophy.
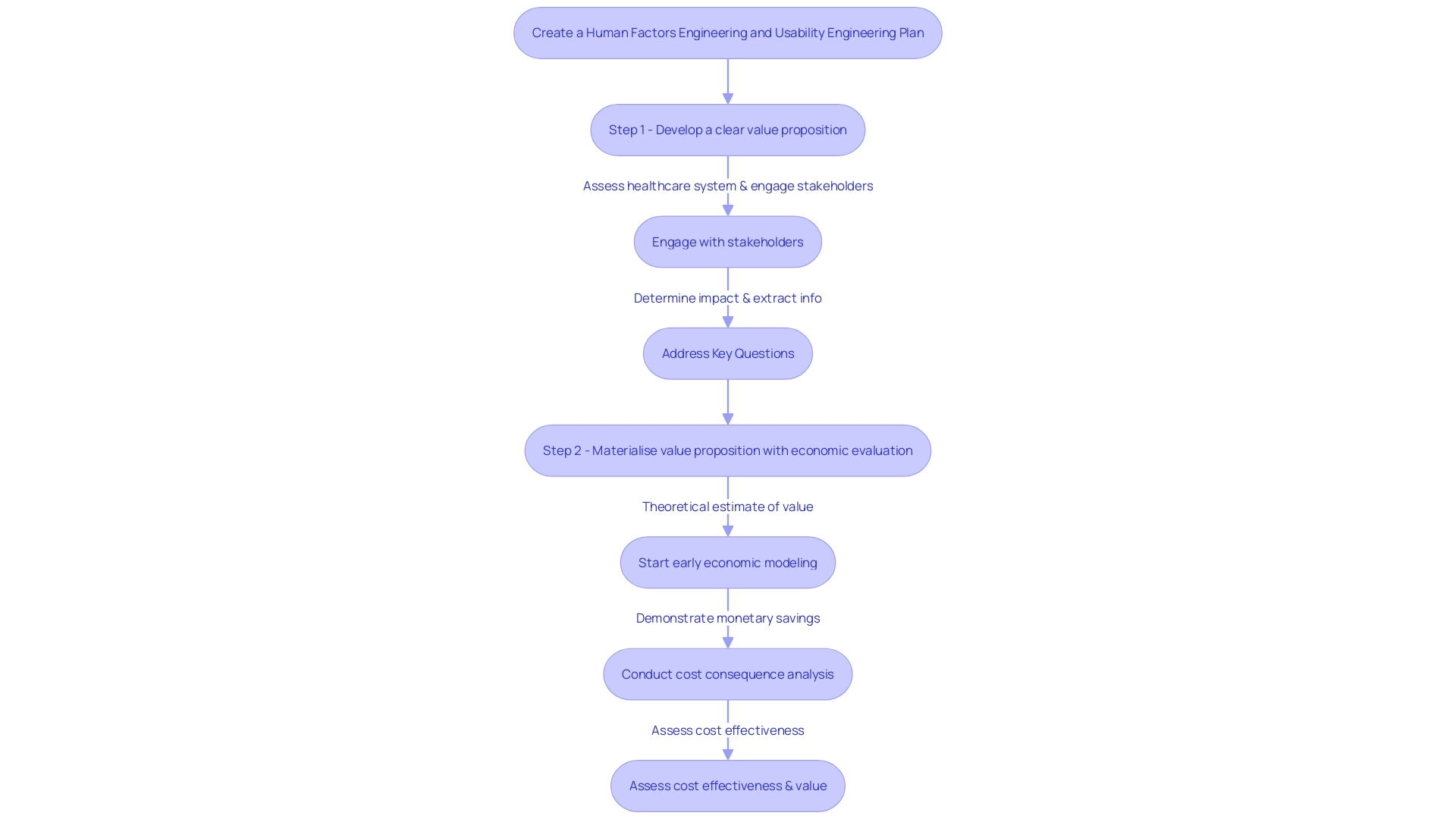
Commercialization is the ultimate goal, where a healthcare instrument transitions from concept to market-ready product. It encompasses a comprehensive understanding of the healthcare system, stakeholder needs, and the value proposition of the equipment. Engaging with clinicians, patients, and others who will interact with the technology, along with analyzing published evidence, informs a robust go-to-market strategy. An economic evaluation further materializes this strategy, demonstrating the product's cost-effectiveness and value to payers.
As Perry Parendo suggests in his insights on new product development, balancing patient and project risks is crucial. Comprehending different development approaches—from sequential to flexible—and customizing them to the healthcare equipment industry's distinct requirements can reduce project risks and enhance the probability of achievement.
Finally, the voyage of device development is a testament to the collective effort of dedicated individuals who are committed to enhancing healthcare outcomes. The process is not a gamble but a calculated progression through well-managed stages, where the confluence of human factors, usability engineering, and strategic commercialization efforts can lead to the creation of extraordinary medical solutions.
Conclusion
To conclude, Human Factors and Usability Engineering (HF&UE) are vital in the design and development of medical devices. By integrating these principles, designers can create functional and intuitive devices that reduce the risk of user error and improve healthcare delivery. Comprehensive planning, collaboration, and user-centered design are essential for ensuring the safety, effectiveness, and adaptability of medical devices in the ever-changing healthcare landscape.
Understanding the importance of human factors in medical device design enhances usability, effectiveness, and safety. By considering the needs of all users, including patients and healthcare professionals, designers can mitigate risks and drive market innovation.
The integration of HF&UE involves key steps such as user research, user-centered design, regulatory compliance, and usability testing. Flexibility in design is crucial to adapt to future changes in healthcare technology.
Regulatory compliance and standards are indispensable in medical device development. Compliance with guidelines from organizations like the FDA and IEC 62366 ensures the safety and effectiveness of devices.
Innovative design principles minimize user errors and enhance patient safety. Robust cybersecurity measures and effective user training play a crucial role in achieving this goal.
Creating a comprehensive human factors plan requires meticulous planning, collaboration, and multidisciplinary approaches. Prioritizing usability and safety ensures that devices meet the needs of all end-users.
Integrating human factors and usability engineering into medical device development involves involving end-users from the earliest stages of design, iterative design and usability testing, and a strategic approach that considers the impact on users and the value the device brings.
The journey of medical device development requires passion and commitment to improving healthcare outcomes. By incorporating human factors, usability engineering, and strategic commercialization efforts, extraordinary medical solutions can be created.




