Introduction
The landscape of clinical investigations for medical devices is evolving rapidly, driven by regulatory advancements and technological innovations. The FDA's comprehensive guidance on pivotal clinical trials underscores the critical importance of meticulous study design, emphasizing factors such as:
- Endpoint selection
- Population representation
- Statistical rigor
As organizations navigate these complexities, the incorporation of risk management strategies becomes essential, ensuring patient safety and data integrity throughout the trial process. Furthermore, engaging stakeholders and leveraging technology are pivotal in enhancing collaboration and operational efficiency.
This article delves into the key elements that contribute to successful clinical trial execution, offering insights into best practices that align with regulatory expectations and foster meaningful outcomes in the medical device sector.
Understanding FDA Guidance on Design Considerations for Pivotal Clinical Investigations
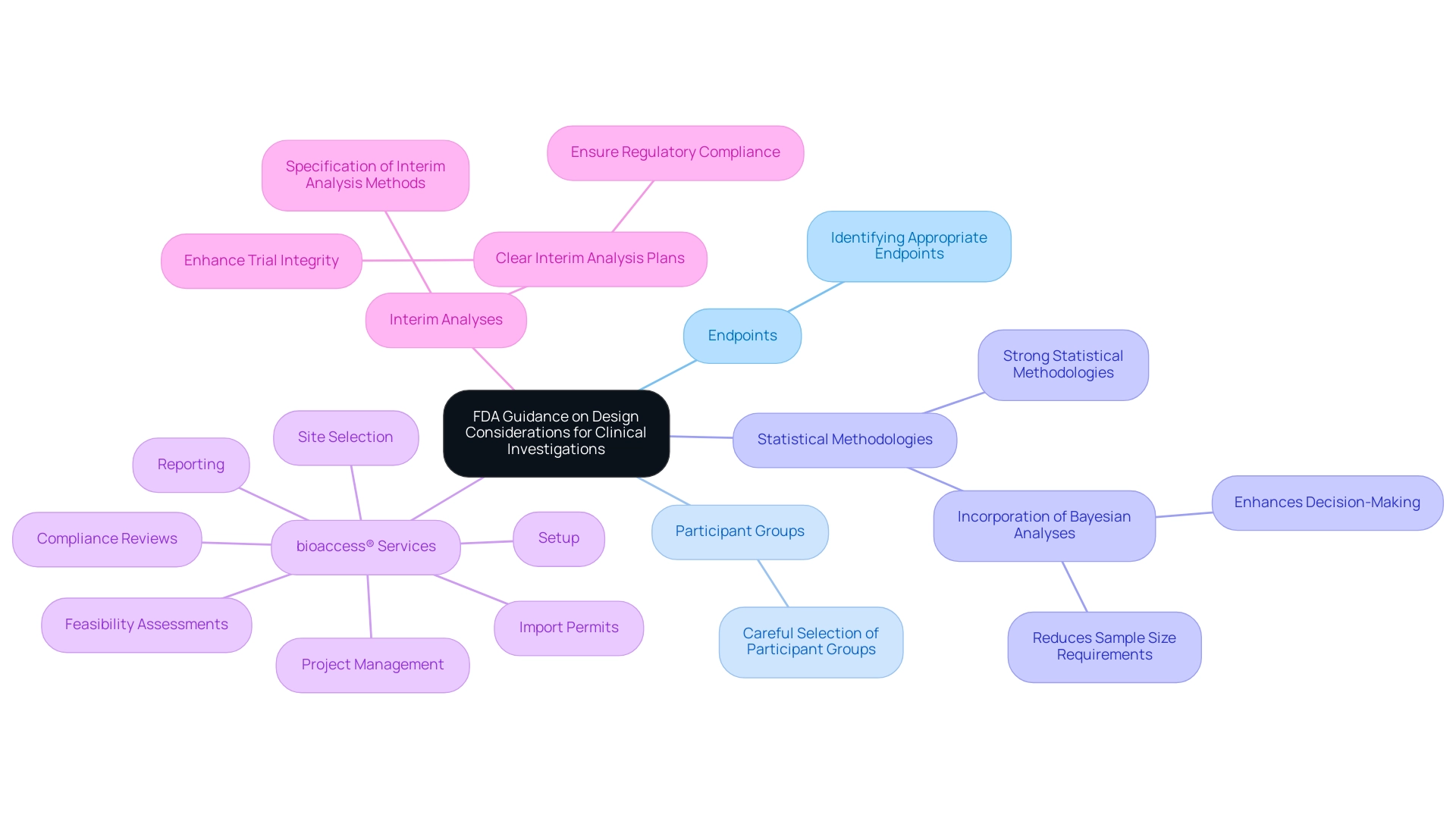
The FDA has provided comprehensive guidance on design considerations for pivotal clinical investigations for medical devices, emphasizing several crucial factors, such as:
- Identifying appropriate endpoints
- Careful selection of participant groups
- Strong statistical methodologies in research design
Researchers are urged to organize their investigations in a manner that yields valid and reliable data, demonstrating the safety and effectiveness of the device in question. Notably, the incorporation of Bayesian analyses can enhance decision-making processes, as the predictive probability at an interim point can serve as a rule for stopping a trial, potentially reducing sample size requirements and optimizing resources.
Reviewing key documents, such as the 'FDA Guidance for Industry and FDA Staff: design considerations for pivotal clinical investigations for medical devices,' is crucial for aligning research protocols with regulatory expectations. Adherence to these guidelines not only fortifies the integrity of the research but also fosters confidence among stakeholders, including regulatory bodies and project sponsors. As Leslie Kux, Assistant Commissioner for Policy, stated, 'Comprehensive planning and transparency in research designs are vital for achieving favorable outcomes.'
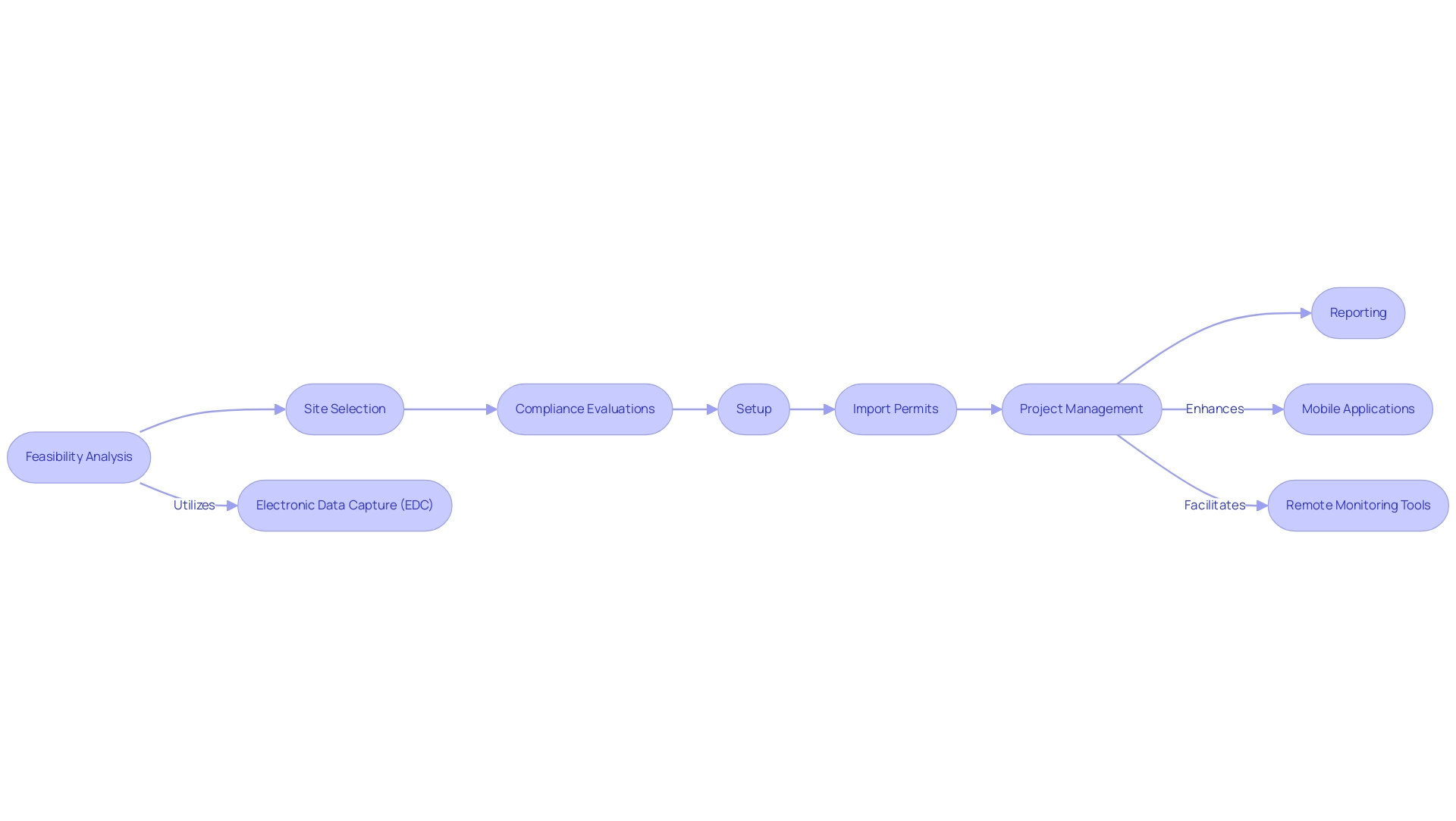
Moreover, with more than 20 years of experience in Medtech, bioaccess® provides extensive management services for research projects customized to address these design factors, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Their proficiency in overseeing different research types, such as Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Investigations, positions them as an essential partner for U.S. medical device firms navigating intricate trials in Latin America. Furthermore, the case study titled 'Interim Analyses in Clinical Trials' emphasizes the FDA's suggestion to outline interim analysis methods in study designs, highlighting the significance of clear interim analysis plans in improving study integrity and ensuring regulatory compliance.
This emphasis on regulatory adherence is crucial for aligning bioaccess®'s services with FDA guidelines, further solidifying their position as a reliable ally in the research landscape.
Key Elements of Effective Clinical Trial Design for Medical Devices
Creating effective medical studies for devices necessitates incorporating design considerations for pivotal clinical investigations for medical devices, as careful consideration of several essential factors can greatly impact results. A fundamental step in this process is the precise definition of primary and secondary endpoints, ensuring they are not only clinically relevant but also measurable. According to Johnson & Albert, "Bayesian analysis of ordinal outcomes using latent variables can further refine endpoint definitions and enhance the interpretability of results."
Choosing a research population that precisely represents the intended application of the device is equally crucial; participants should be typical to provide valuable safety and efficacy information.
At bioaccess®, we provide comprehensive clinical study management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
These are critical components for successful execution. With over 20 years of experience in Medtech, our expertise spans:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
This reflects our commitment to navigating the complexities of medical device investigations in Latin America with flexibility and precision.
Statistical considerations are paramount in study design, particularly in determining appropriate sample sizes and selecting robust statistical methods for data analysis. Notably, the posterior probability that the adverse event rate is greater than 0.4 after observing one adverse event in 10 patients is about 0.04, highlighting the significance of careful endpoint selection and monitoring. The FDA's recent recommendations highlight the necessity of specifying methods for analyzing interim results prior to study initiation, emphasizing the importance of obtaining regulatory agreement.
Furthermore, incorporating appropriate controls, blinding, and randomization is essential to mitigate bias and ensure the integrity of the study findings.
The implications of multiplicity in medical studies also require attention; performing numerous hypothesis tests can heighten the risk of false-positive results. The article on multiplicity emphasizes the importance of controlling the false positive error rate and suggests methods for reporting and adjusting significance levels to mitigate this risk. Therefore, researchers must implement strategies for controlling the false positive error rate, such as adjusting significance levels and carefully reporting results.
By thoughtfully integrating the design considerations for pivotal clinical investigations for medical devices into the study design, alongside the expert guidance of bioaccess®, researchers enhance the likelihood of achieving valid results that meet regulatory expectations, ultimately leading to improved patient outcomes and assisting companies in navigating towards successful acquisitions.
Incorporating Risk Management Strategies in Clinical Investigations
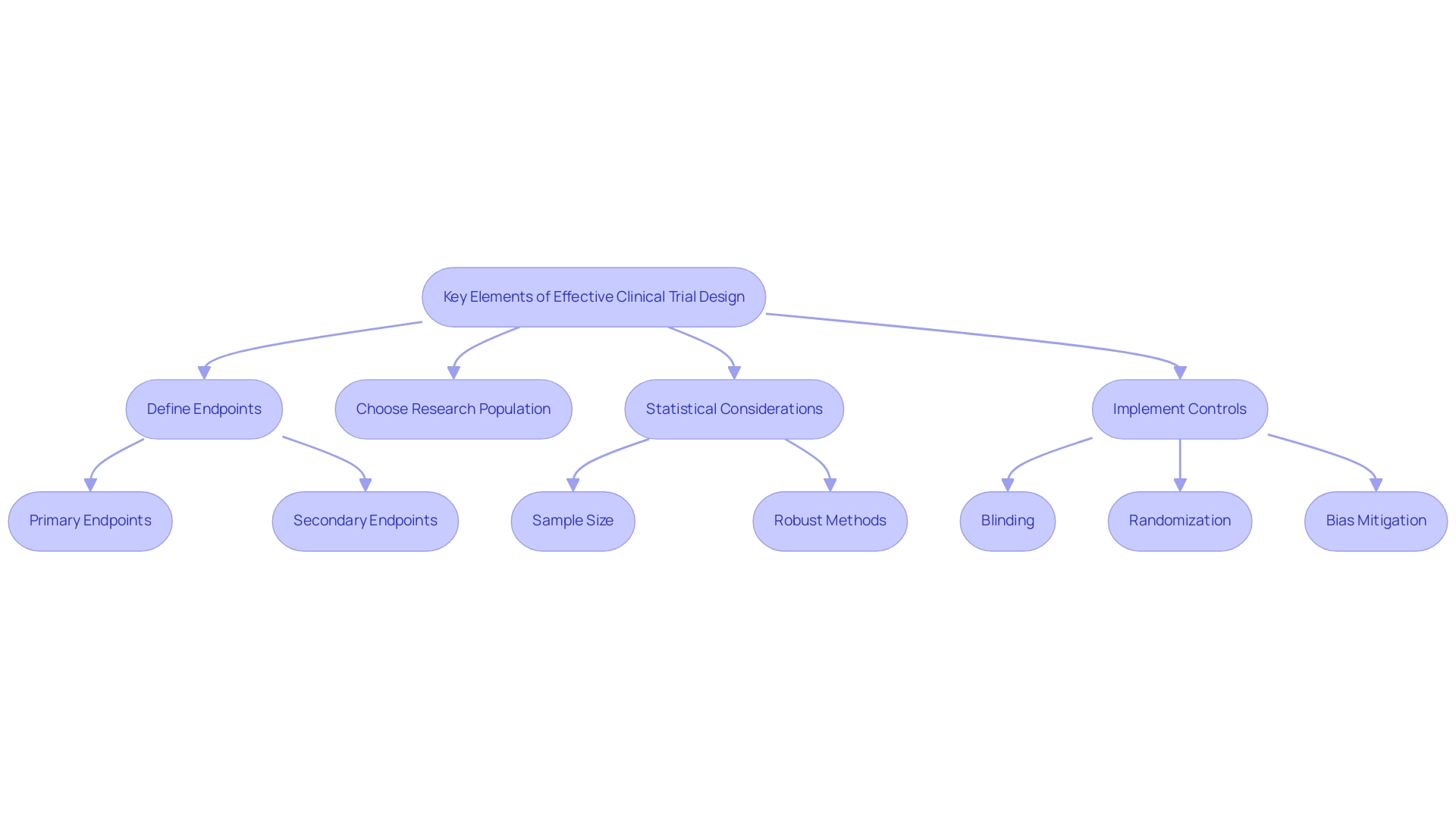
Integrating risk management strategies into medical investigations is essential for addressing design considerations for pivotal clinical investigations for medical devices, ensuring patient safety and maintaining the integrity of collected data. A thorough risk evaluation should be the first step, focused on identifying potential dangers related to both the medical device and the research process. This assessment must evaluate the likelihood and impact of the identified risks.
Recent findings emphasize common obstacles encountered by clinical research locations, including:
- Lack of time for investigators (70.0%)
- Financial negotiation delays (28.3%)
- Absence of trained personnel (28.3%)
- Recruitment challenges (26.1%)
This highlights the urgency of effective risk management strategies.
To tackle these issues, our service offerings include:
- Feasibility assessments
- Selection of research sites and principal investigators (PIs)
This ensures that projects are organized efficiently and in accordance with country-specific requirements. This includes detailed processes for trial set-up and approval by ethics committees and health ministries, as well as obtaining necessary import permits and nationalization of investigational devices. Once risks are categorized, researchers can develop proactive mitigation strategies tailored to address them.
Regular monitoring and documentation of risk management activities throughout the study are critical, allowing for timely adaptations in response to any emerging issues. As pointed out by Dr. Gregory Levin, Associate Director for Statistical Science and Policy, effective risk management necessitates careful consideration of analysis approaches—encompassing treatment discontinuation handling and the integration of information from multiple trials.
Moreover, continued support from regulatory bodies is vital for the successful adoption and implementation of Risk-Based Quality Management (RBQM), providing a framework within which these risk management strategies operate. Implementing these strategies not only safeguards participants but also enhances the credibility of research findings. By focusing on design considerations for pivotal clinical investigations for medical devices, researchers can prioritize risk evaluation and navigate complex challenges while upholding the highest standards of patient safety and information integrity.
For instance, our reporting services include:
- Study status updates
- Inventory management
- Tracking serious and non-serious adverse events
All of which comply with privacy legislation and regulatory requirements, ensuring data integrity and confidentiality as illustrated in our case study on data management, statistics, and reporting. To talk about how we can assist with your research requirements, SCHEDULE A MEETING with us today.
Engaging Stakeholders and Building Collaborative Relationships
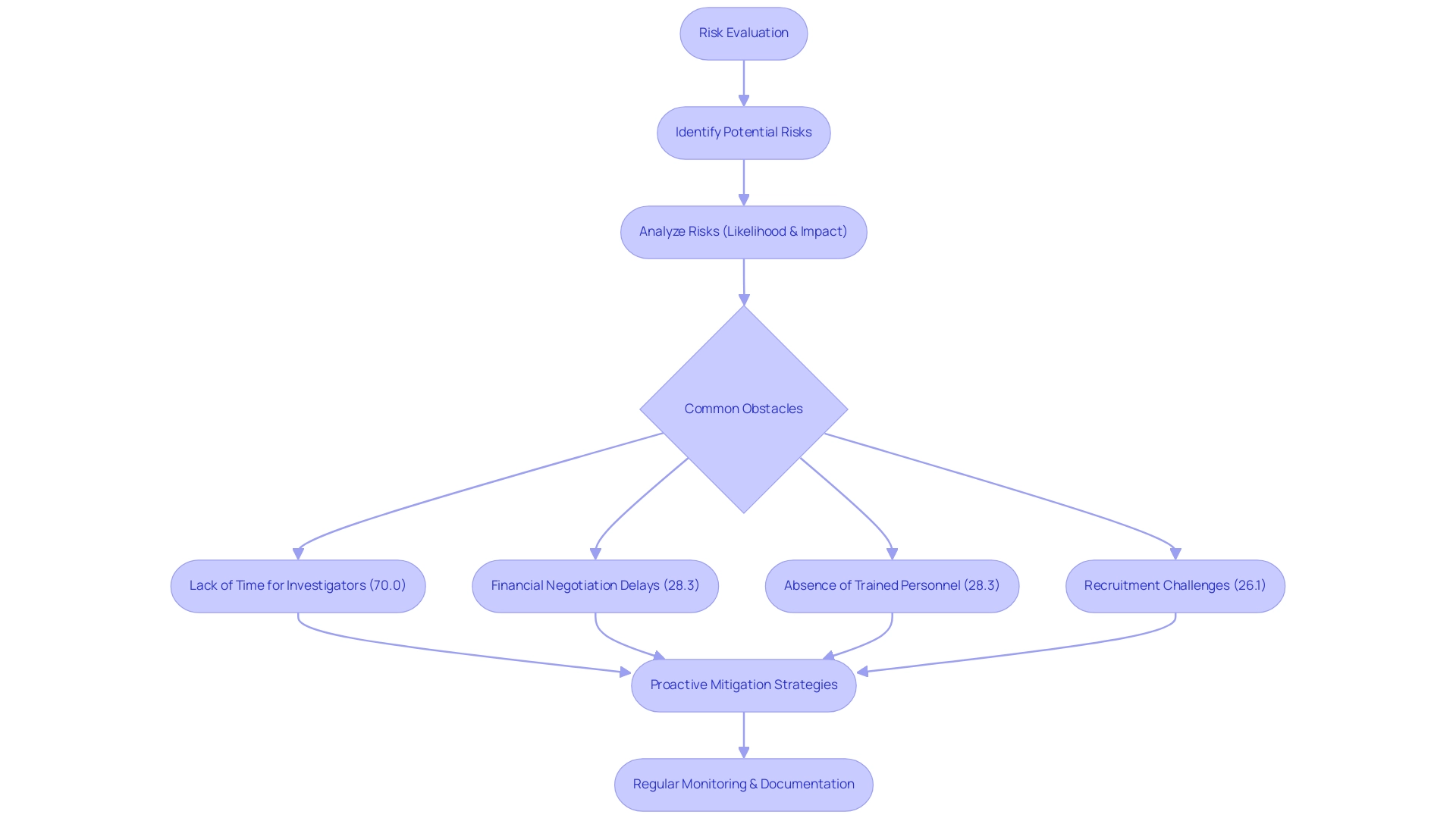
Involving stakeholders and cultivating cooperative relationships are vital elements for the success of research investigations. Effective communication with all involved parties—investigators, sponsors, regulatory bodies, and ethics committees—should be a top priority. Our comprehensive clinical trial management services encompass:
- Feasibility analysis
- Site selection
- Compliance reviews
- Trial setup
- Reporting on investigation status, inventory, as well as serious and non-serious adverse events, ensuring that each investigation is thoroughly supported.
Data indicates that 50% of research teams face difficulties in finding suitable times and locations for meetings, which can hinder engagement. Maintaining regular updates and transparent discussions regarding research progress, challenges, and outcomes cultivates a collaborative environment that can significantly enhance project execution. Moreover, our services also address the import permits and nationalization of investigational devices, further streamlining the process.
As demonstrated in the Veterans Response to Dose in Chiropractic Therapy Clinical Trial, communication and feedback played a crucial role in adjusting protocols to streamline processes. A case analysis titled 'Impact of Stakeholder Engagement on Research Rigor' found that the involvement of stakeholders enhanced the rigor of asthma research, making outcomes more meaningful and applicable to underrepresented populations. Engaging stakeholders during the planning phase can yield valuable insights that enhance research design and implementation, serving as crucial design considerations for pivotal clinical investigations for medical devices, and directly contributing to improved patient-centered outcomes.
Successful partnerships not only facilitate smoother operational processes but also lead to better results, which can have a positive impact on local economies through job creation, economic growth, and healthcare improvement. A researcher, who received her BSN from Minnesota State University, Mankato, and her MA with a focus in medical ethics from Duke University, noted,
The research was completed. We exceeded expectations…We recruited more than we expected, we enrolled more than we expected.
It was phenomenal from that frame, exemplifying the tangible benefits of stakeholder engagement.
Utilizing Technology to Enhance Clinical Trial Efficiency
The integration of technology in medical studies is crucial for improving efficiency and ensuring data integrity. Our extensive research management services encompass:
- Feasibility analysis
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting, including review and feedback on research documents to meet country requirements
A systematic map reviewed 105 studies, revealing the transformative potential of digital tools for recruitment and retention, underscoring their essential role in contemporary research.
According to CISCRP, a significant percentage of clinical trial participants utilize various types of technology, which further highlights the impact of these tools. Researchers are increasingly encouraged to implement electronic data capture (EDC) systems, which streamline data collection and management, ultimately leading to improved operational workflows. Mobile applications also play a crucial role by facilitating participant engagement and adherence to protocols through timely reminders and real-time feedback.
Furthermore, remote monitoring tools have emerged as effective solutions for overseeing activities, significantly reducing the need for frequent on-site visits, thereby conserving valuable time and resources. The combination of these technologies, together with our skilled project management, not only improves the efficiency of research studies but also leads to enhanced information quality. Reporting on study status and serious and non-serious adverse events is a critical aspect of our services, ensuring transparency and compliance throughout the research process.
For example, the application of real-life information in medical studies has shown significant enhancements in recruitment methods and overall study efficiency, highlighting the considerable advantages of embracing digital technologies. As we transition into 2024, the newest innovations in electronic information collection will keep influencing the environment of research studies, with ongoing discussions emphasizing their effect on efficiency and reliability. As noted by experts, incorporating artificial intelligence and machine learning algorithms will further enhance the analysis of big data, as Mak states, 'improving analysis of big data when using AI and ML algorithms,' paving the way for more effective and responsive clinical trials.
Conclusion
The evolving landscape of clinical investigations for medical devices necessitates a meticulous approach to study design, emphasizing the importance of:
- Endpoint selection
- Population representation
- Statistical rigor
Adhering to the FDA's guidance on pivotal clinical trials not only strengthens the validity of research findings but also enhances stakeholder confidence. By integrating risk management strategies and engaging stakeholders throughout the process, researchers can navigate potential challenges while prioritizing patient safety and data integrity.
Furthermore, the incorporation of innovative technologies plays a crucial role in streamlining trial operations and improving data quality. The utilization of:
- Electronic data capture systems
- Mobile applications
- Remote monitoring tools
significantly enhances recruitment, retention, and overall trial efficiency. These advancements not only facilitate compliance with regulatory expectations but also foster a collaborative environment among all parties involved.
Ultimately, successful clinical trial execution hinges on a comprehensive understanding of these key elements and the proactive adoption of best practices. By aligning with regulatory guidelines and leveraging the expertise of clinical trial management services, organizations can significantly improve the likelihood of achieving meaningful outcomes, paving the way for advancements in the medical device sector and better patient care in the future.
Frequently Asked Questions
What are the key design considerations for pivotal clinical investigations for medical devices according to the FDA?
The key design considerations include identifying appropriate endpoints, careful selection of participant groups, and employing strong statistical methodologies in research design.
Why is it important to select appropriate endpoints in clinical investigations?
Selecting appropriate endpoints is crucial as they must be clinically relevant and measurable to yield valid and reliable data regarding the safety and effectiveness of the device.
How can Bayesian analyses improve clinical trial decision-making?
Bayesian analyses can enhance decision-making by providing predictive probabilities at interim points, which can serve as criteria for stopping a trial, potentially reducing sample size requirements and optimizing resources.
What role does bioaccess® play in managing clinical studies for medical devices?
Bioaccess® offers comprehensive clinical study management services, including feasibility assessments, site selection, compliance reviews, project management, and reporting, tailored to address design considerations in medical device investigations.
What types of studies does bioaccess® specialize in?
Bioaccess® specializes in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
What is the significance of interim analysis plans in clinical trials?
Interim analysis plans are significant as they enhance study integrity and ensure regulatory compliance by outlining methods for analyzing interim results prior to the initiation of the study.
Why is participant selection important in clinical investigations?
Participant selection is important because the research population should accurately represent the intended application of the device to provide valuable safety and efficacy information.
What statistical considerations should researchers keep in mind during study design?
Researchers should determine appropriate sample sizes, select robust statistical methods for data analysis, and incorporate controls, blinding, and randomization to mitigate bias and ensure integrity.
How does the concept of multiplicity affect medical studies?
The concept of multiplicity can heighten the risk of false-positive results due to performing numerous hypothesis tests, necessitating strategies to control the false positive error rate.
What is the overall goal of integrating design considerations in clinical investigations?
The overall goal is to enhance the likelihood of achieving valid results that meet regulatory expectations, ultimately improving patient outcomes and assisting companies in successful acquisitions.

