Overview
The article focuses on best practices for ensuring cost efficiency in clinical trials conducted in Latin America, highlighting strategies to optimize resources and enhance study outcomes. It emphasizes the region's advantages, such as lower operational costs and reliable patient engagement, while also addressing challenges like regulatory hurdles and the need for tailored health policies to maintain efficiency, ultimately supporting the notion that strategic planning and local partnerships are crucial for successful trials.
Introduction
In the dynamic landscape of clinical research, cost efficiency emerges as a crucial factor that can determine the success or failure of trials. With increasing financial pressures and the necessity for high-quality outcomes, organizations are turning their attention to innovative strategies that streamline operations and enhance resource allocation. Particularly in Latin America, where budgetary constraints are often pronounced, the focus on cost-effective trial management is paramount.
Recent collaborations, such as the partnership between bioaccess™ and Caribbean Health Group, highlight the region's potential to become a hub for clinical trials, promising not only economic benefits but also improved healthcare outcomes.
As the clinical trials market evolves, understanding how to navigate challenges while leveraging the unique advantages of Latin America becomes essential for organizations aiming to optimize their research investments.
Understanding Cost Efficiency in Clinical Trials
Cost efficiency in clinical studies is pivotal for conducting research within budgetary limits while ensuring the highest quality and integrity of results. It entails a comprehensive examination of operational expenses, resource distribution, and project timelines to enable efficient study implementation. In Latin America, where financial resources can be constrained, a keen focus on the cost efficiency of clinical trials in Latin America becomes essential for successful management of studies.
The recent partnership between bioaccess™ and Caribbean Health Group, revealed on March 29, 2019, during a meeting at PROCOLOMBIA's office in Miami, FL, aims to establish Barranquilla as a prominent location for medical research. Backed by Colombia's Minister of Health, this initiative aims to draw more research projects to the region, thereby enhancing local economic conditions. By implementing strategies aimed at reducing costs without sacrificing quality, researchers can significantly enhance the probability of research success.
This method not only improves operational efficiency but also aligns with the current trend in the medical research market, where strategic collaborations and acquisitions are increasingly prioritized—accounting for 65% of total investment activity in 2023. GlobalCare Clinical Trials' partnership with bioaccess™ has already demonstrated remarkable results, achieving over a 50% reduction in recruitment time and 95% retention rates. Such collaborations offer extra resources and knowledge, enabling navigation of the complexities of medical research while adhering to budget constraints.
As mentioned in the case study 'Regions for Cheaper Medical Studies,' certain areas, including Latin countries, provide economical choices for research, which emphasizes the cost efficiency of clinical trials in Latin America, while also posing challenges such as political instability and differing regulatory procedures that must be navigated carefully to ensure successful outcomes.
Leveraging Latin America's Advantages for Cost-Effective Trials
Latin nations are distinguished as a region that greatly contributes to the cost efficiency of clinical trials in Latin America. Experts indicate that dropout rates here are only one-third of those observed in the U.S. and EU, showcasing the reliability of patient engagement in this area. As stated by Dushyanth Surakanti, Founder & CEO of Sparta Biomedical, 'The dedication of patients in Latin regions is unmatched, which is essential for the success of clinical studies.'
The lower operational costs—encompassing labor, facility, and regulatory expenses—further solidify the cost efficiency of clinical trials in Latin America, making it more attractive for research endeavors. Countries like Brazil, Mexico, and Argentina boast diverse patient populations, which not only enrich the participant demographic but also facilitate quicker recruitment processes and reduce overall timelines. Recent reports emphasize that the cost efficiency of clinical trials in Latin America, especially in Brazil and Mexico, is considerably lower than that in the U.S., making these nations even more attractive for sponsors.
Furthermore, the study emphasized the urgent need for tailored health policy interventions to improve treatment accessibility and optimize resource allocation for stroke care in Latin regions. By strategically choosing research study locations in this region, sponsors can enhance their budgets, contributing to the cost efficiency of clinical trials in Latin America while ensuring that high-quality data gathering remains a priority. The partnership with LATAM CRO specialists, along with extensive research management services—including:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
positions Latin America as an attractive option for organizations seeking to achieve the cost efficiency of clinical trials in Latin America while promoting local economic growth and healthcare enhancement.
With a proven background of over 20 years in Medtech, bioaccess® has the expertise and tailored approach you need to guide your company towards successful trial outcomes.
The Role of CROs in Enhancing Cost Efficiency
Contract Research Organizations (CROs) play an essential role in improving the cost efficiency of clinical trials in Latin America by offering specialized knowledge that encompasses regulatory compliance, data management, and patient recruitment. By outsourcing to CROs, sponsors can significantly enhance the cost efficiency of clinical trials in Latin America by reducing overhead costs tied to staffing and infrastructure, allowing them to allocate resources more effectively. A notable advantage is CROs' established relationships with local regulatory authorities, which can expedite the approval process and reduce potential delays.
This is especially clear in Colombia, where the total IRB/EC and MoH (INVIMA) review process requires only 90-120 days, offering a substantial benefit for first-in-human studies. Additionally, the cost efficiency of clinical trials in Latin America is highlighted by Colombia's ability to provide over 30% savings compared to similar experiments in North America or Western Europe, along with a healthcare system recognized as one of the finest in Latin America and the world by the World Health Organization. The partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a premier location for research studies, backed by the Colombian Minister of Health, which further increases the attractiveness for CROs and sponsors alike.
Additionally, Colombia's population exceeds 50 million, with approximately 95% of the population covered by universal healthcare, which supports robust patient recruitment capabilities. The R&D tax incentives offered in Colombia, featuring:
- A 100% tax deduction for investments in science, technology, and innovation
- A 25% tax discount
- A 50% future tax credit
provide additional financial benefits for medical device firms contemplating studies in the region. These elements encourage a more efficient method of managing research studies, which contributes to the cost efficiency of clinical trials in Latin America, resulting in significant savings and enhanced patient recruitment results.
Sofpromed, for example, seeks to assist small biotech firms carry out research studies at competitive prices, demonstrating the increasing significance of CROs for these businesses. According to Straits Research, the worldwide market revenue in medical studies is set to hit USD 47.58 billion by 2024 and is anticipated to rise to USD 83.51 billion by 2033. This highlights the significance of utilizing CROs for competitive benefit in this changing environment, especially in Latin regions, where recent trends show that the cost efficiency of clinical trials in Latin America is becoming more crucial for biotech companies managing the intricacies of research studies.
Navigating Challenges to Maintain Cost Efficiency
Despite the unique benefits that Latin regions provide for medical studies, the area faces obstacles that greatly impact the cost efficiency of clinical trials in Latin America. Regulatory hurdles, including protracted approval processes and inconsistent compliance requirements across various countries, can lead to delays and escalated costs. Moreover, patient recruitment presents its own set of difficulties, as cultural disparities and uneven access to healthcare create barriers.
A troubling statistic shows that 85% of patients in Latin regions stay uninformed about their choices for engaging in research studies at the moment of diagnosis, highlighting a significant chance for enhanced participation. To effectively navigate these challenges, engaging local experts who are well-versed in the regional regulatory landscape is essential in facilitating smoother processes. Significantly, the partnership between bioaccess™ and the Caribbean Health Group seeks to establish Barranquilla as a premier location for medical studies in Latin America, backed by Colombia's Minister of Health.
This partnership highlights the significance of nurturing local relationships to improve patient recruitment initiatives, ultimately promoting the cost efficiency of clinical trials in Latin America. Additionally, Brazil generated 1561 citable documents in 2020, representing 45.8% of the region's total, highlighting its important role in research. The recent enactment of Law 14.874/24, aimed at simplifying the evaluation process for research studies in Brazil, exemplifies efforts to strengthen the research environment.
As Federico Waisberg aptly states, in this time of change in LATAM, a 'res, non verba' ('actions, not words') approach should be the motto for advancing cancer research studies. The challenge of recruiting patients from high-income countries and the necessity of creating local innovative projects remain critical considerations for the future. Significantly, the partnership has accomplished more than a 50% decrease in recruitment time and an impressive 95% retention rate, demonstrating the effectiveness of localized strategies in improving research outcomes.
Best Practices for Optimizing Cost Efficiency in Clinical Trials
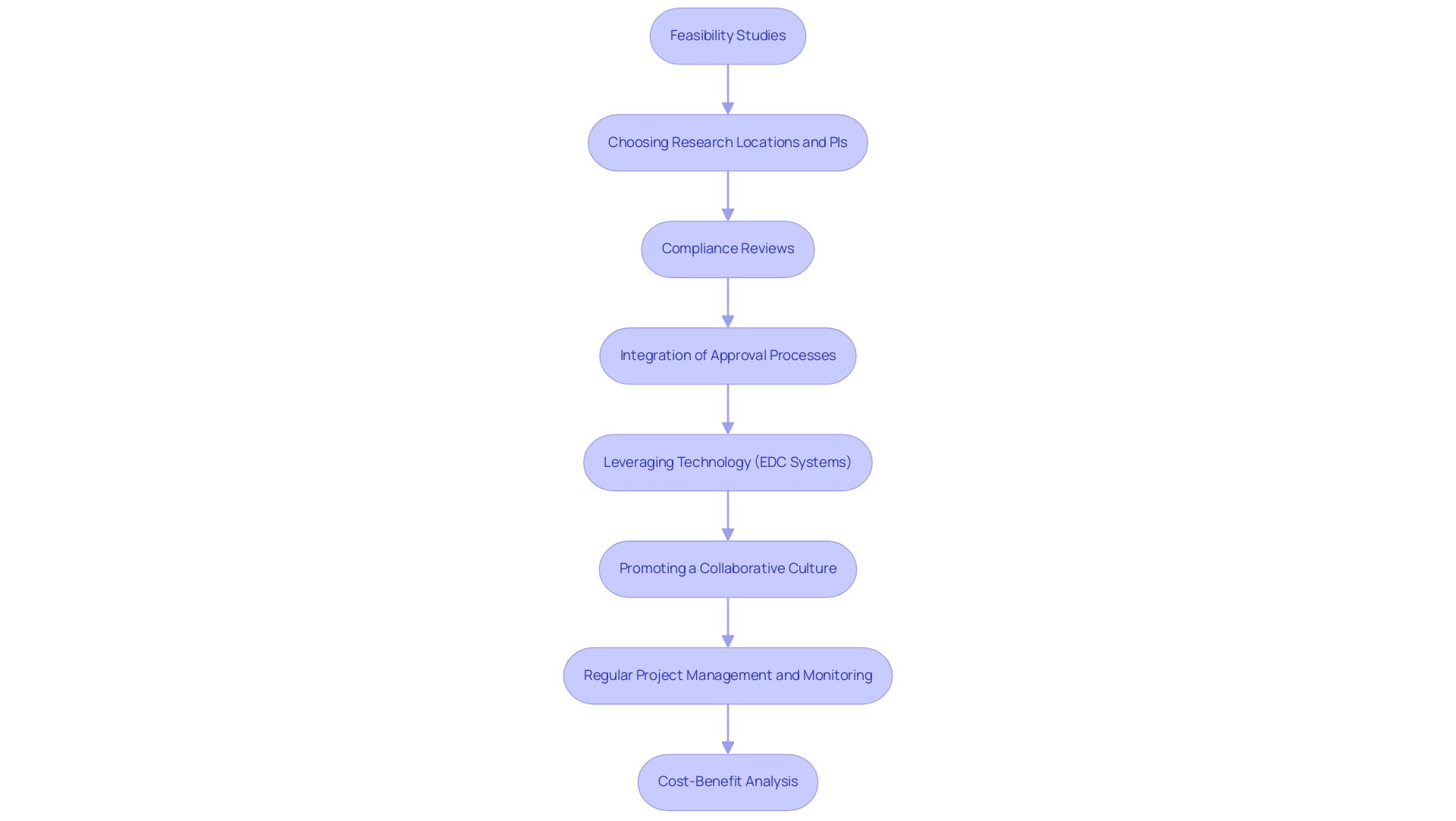
To enhance the cost efficiency of clinical trials in Latin America, implementing a comprehensive range of best practices is essential. This involves:
- Performing feasibility studies
- Choosing suitable research locations and principal investigators (PIs) before the commencement, which aids in identifying possible challenges and optimizing processes efficiently.
Additionally, performing compliance reviews on study documents ensures adherence to local regulations, further enhancing efficiency.
The experimental setup and approval processes, including obtaining necessary ethics committee and health ministry approvals, are critical steps that must be integrated into the planning phase. Leveraging technology—specifically electronic data capture (EDC) systems—can significantly minimize data entry errors and improve the overall quality of data collected. This technological progress not only saves time but also aids in expense reductions throughout the process.
As preference-weighted quality-of-life scores are typically gathered every three months during studies, this data can be pivotal in assessing the value of treatments. Promoting a collaborative culture among team members and stakeholders fosters innovative solutions that enhance operational efficiency. Furthermore, regular project management and monitoring, including comprehensive reporting on study status and serious and non-serious adverse events, are essential to ensuring the cost efficiency of clinical trials in Latin America throughout the study duration.
A cost-benefit analysis serves as a method to measure financial costs of specific budget items against their potential benefits, ensuring that each resource is utilized effectively. As Elizabeth Olsen, Director of Scientific & Health Science Policy Initiatives, notes, these practices are vital for maximizing the cost efficiency of clinical trials in Latin America, ensuring that every resource is utilized to its fullest potential while also contributing to job creation, economic growth, and healthcare improvement in the local economies.
Conclusion
The landscape of clinical trials in Latin America is evolving, driven by a strong emphasis on cost efficiency and strategic collaborations. By focusing on operational excellence and leveraging local resources, organizations can significantly enhance their research investments. The partnership between bioaccess™ and Caribbean Health Group exemplifies how regional initiatives can attract more clinical research projects, ultimately benefiting both the economy and healthcare outcomes.
Latin America offers unique advantages, including lower operational costs and high patient engagement rates, which contribute to more efficient trial management. As highlighted, the combination of diverse patient populations and reduced dropout rates positions the region as an attractive option for trial sponsors. However, navigating the complexities of local regulations and cultural disparities is crucial for maximizing these benefits.
Implementing best practices such as conducting thorough feasibility studies and utilizing technology can further streamline trial processes. Engaging local experts ensures compliance and fosters effective patient recruitment, addressing the challenges that may arise. As the clinical trials market continues to grow, especially with the increasing role of Contract Research Organizations (CROs), the potential for Latin America to emerge as a leading hub for cost-effective trials becomes increasingly evident.
In conclusion, the collective efforts to enhance cost efficiency in clinical trials not only optimize resource allocation but also pave the way for improved healthcare solutions. As organizations embrace innovative strategies and partnerships, the future of clinical research in Latin America looks promising, with the potential to deliver high-quality outcomes while supporting economic growth and community health.
Frequently Asked Questions
Why is cost efficiency important in clinical studies?
Cost efficiency is crucial for conducting research within budgetary limits while ensuring high quality and integrity of results. It involves examining operational expenses, resource distribution, and project timelines to enable efficient study implementation.
What recent initiative aims to enhance medical research in Barranquilla, Colombia?
A partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a prominent location for medical research, supported by Colombia's Minister of Health, to attract more research projects and improve local economic conditions.
How do strategic collaborations impact clinical trials?
Strategic collaborations can significantly enhance operational efficiency and success rates in clinical trials. For example, GlobalCare Clinical Trials' partnership with bioaccess™ achieved over a 50% reduction in recruitment time and 95% retention rates, demonstrating the benefits of shared resources and expertise.
What are some challenges faced in conducting clinical trials in Latin America?
Challenges include political instability and varying regulatory procedures that must be navigated carefully to ensure successful outcomes in clinical trials.
How do dropout rates in Latin America compare to those in the U.S. and EU?
Dropout rates in Latin America are only one-third of those observed in the U.S. and EU, indicating a higher reliability of patient engagement in this region.
What operational cost advantages does Latin America offer for clinical trials?
Latin America has lower operational costs related to labor, facilities, and regulatory expenses, making it more attractive for research endeavors.
Which countries in Latin America are highlighted for their cost efficiency in clinical trials?
Brazil, Mexico, and Argentina are noted for their diverse patient populations, quicker recruitment processes, and significantly lower clinical trial costs compared to the U.S.
What types of studies can benefit from research management services in Latin America?
Research management services in Latin America can support various study types, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies.
How can tailored health policy interventions improve clinical trial outcomes in Latin America?
Tailored health policy interventions can enhance treatment accessibility and optimize resource allocation, ultimately contributing to the cost efficiency of clinical trials while ensuring high-quality data gathering.

