Overview
Best practices for managing multi-country clinical trials emphasize the importance of understanding regional regulations, fostering local collaborations, and leveraging technology to enhance efficiency and compliance. The article supports this by detailing strategies such as engaging regional regulatory consultants, implementing centralized data management systems, and adapting communication to cultural nuances, which collectively improve trial execution and participant recruitment.
Introduction
Conducting clinical trials on a global scale presents a myriad of challenges, particularly in navigating the diverse regulatory, ethical, and cultural landscapes that vary from one country to another. As organizations strive to advance medical research, understanding the intricacies of each region's requirements becomes paramount.
From the initial feasibility studies and site selection to the complexities of informed consent and local compliance, the success of these trials hinges on thorough preparation and effective management. By leveraging local expertise and advanced technologies, researchers can not only streamline processes but also enhance participant engagement and overall trial integrity.
This article delves into the essential strategies for managing multi-country clinical trials, highlighting the importance of collaboration, cultural sensitivity, and technological innovation in achieving successful outcomes.
Navigating Regulatory and Ethical Challenges in Multi-Country Trials
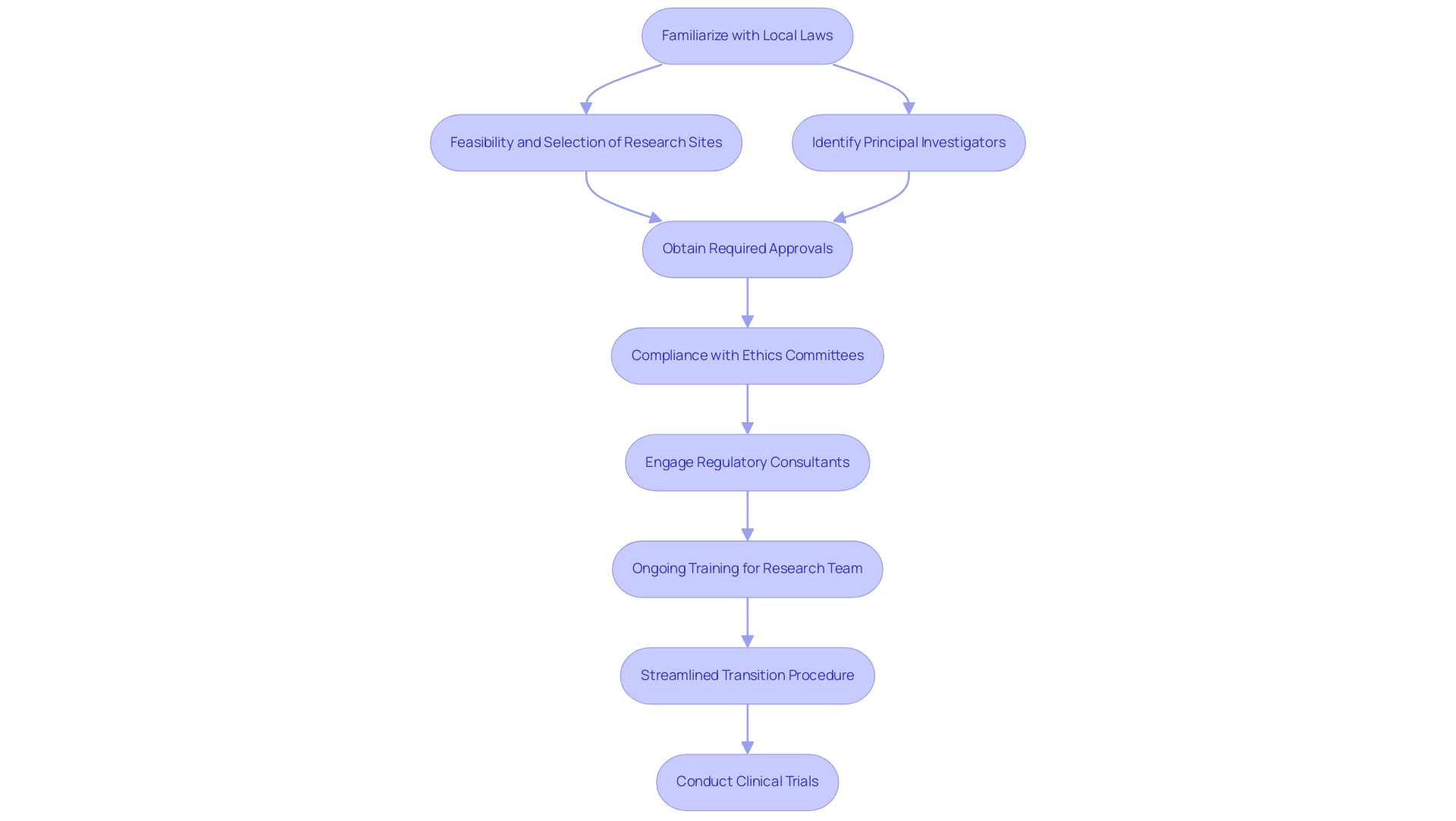
Understanding the distinct regulatory and ethical frameworks that vary by region is among the Best Practices for Managing Multi-Country Clinical Trials. In Colombia, for instance, the feasibility and selection of research sites, along with the identification of principal investigators (PIs), are critical initial steps. Researchers must familiarize themselves with local laws, particularly those governing informed consent processes and reporting obligations.
Compliance with the required approvals from ethics committees, health ministries, and obtaining import permits through INVIMA is essential for regulatory adherence. For example, adherence to the Clinical Trials Regulation (EU) No 536/2014 is mandatory within the European Union, where it establishes rigorous standards for study approval and associated reporting procedures. Significantly, the standard review process may require as long as 106 days if a complete application is necessary, highlighting the time considerations of regulatory compliance in clinical studies.
To navigate these complex regulations effectively, engaging regional Regulatory consultants, such as Ana Criado, who has extensive expertise in biomedical engineering and Regulatory Affairs, can prove invaluable when implementing Best Practices for Managing Multi-Country Clinical Trials. These professionals assist in addressing all ethical considerations and significantly reduce the risk of delays or legal complications. Furthermore, implementing ongoing training for the research team on the Best Practices for Managing Multi-Country Clinical Trials regulations is crucial.
This not only ensures adherence to compliance throughout the process but also fosters a culture of awareness regarding the evolving regulatory landscape. The expedited transition procedure established allows for a minimum dossier review in just 22 days, illustrating how regulatory processes can be streamlined. Additionally, old labels for investigational medicinal products can still be used after the transition until new labels are approved, clarifying ongoing compliance issues that may arise during this period.
By prioritizing education and utilizing local knowledge, such as Katherine Ruiz's insights on regulatory matters for medical devices, studies can be conducted more efficiently and ethically, ultimately enhancing the overall integrity of the research. To learn more about how we can support you in progressing your research studies, SCHEDULE A MEETING.
Strategies for Effective Management of Multi-Country Clinical Trials
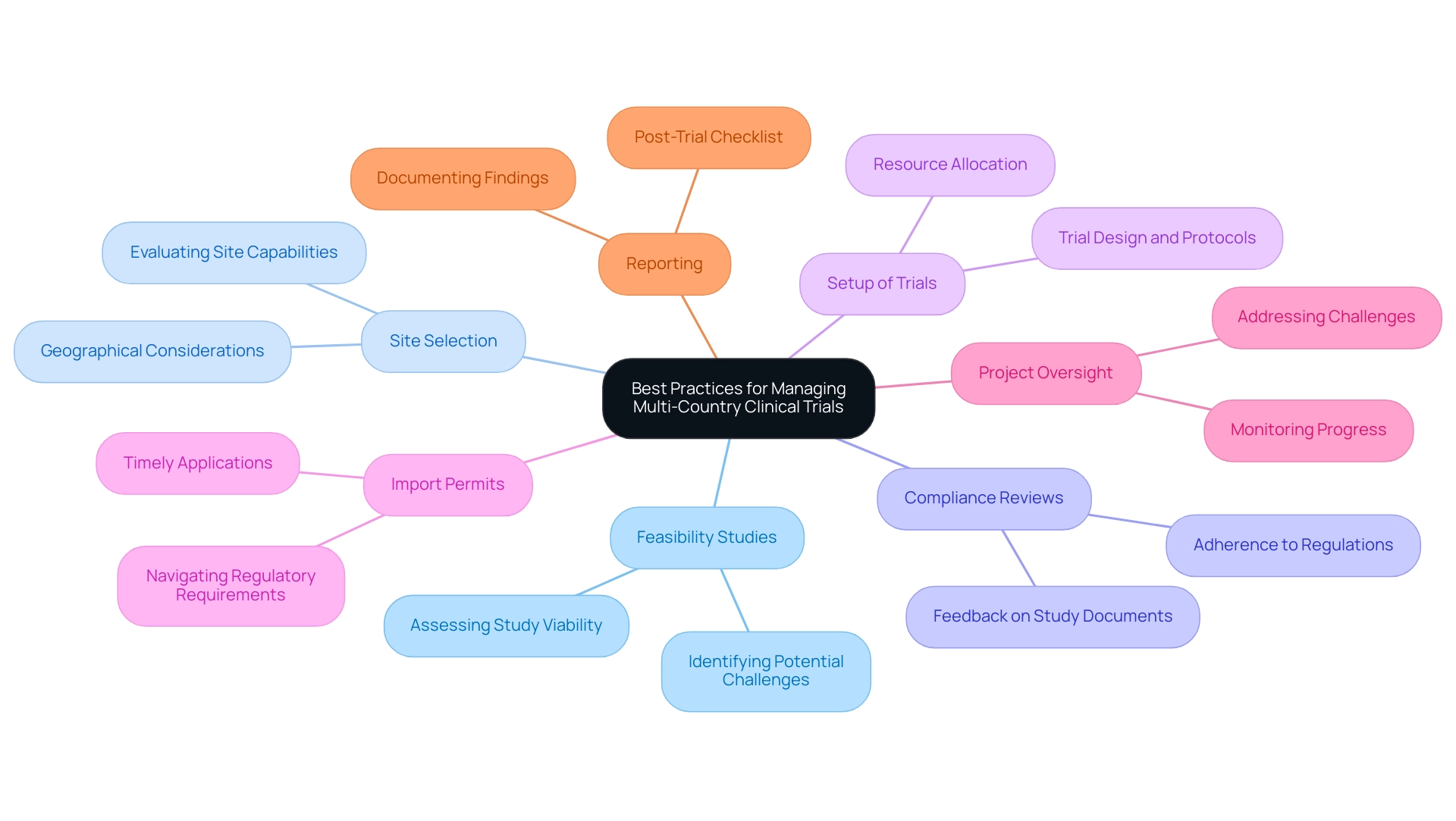
To navigate the complexities of overseeing multi-country research studies effectively, implementing the Best Practices for Managing Multi-Country Clinical Trials is essential. Clear communication channels, alongside well-defined roles and responsibilities, represent the best practices for managing multi-country clinical trials, forming the backbone of successful collaboration across diverse teams. Data shows that almost 80% of all clinical studies face delays mainly because of patient recruitment difficulties, highlighting the essential need for efficiency in operational practices.
To address these challenges, it is essential to follow Best Practices for Managing Multi-Country Clinical Trials, which include thorough services such as:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup of trials
- Import permits
- Project oversight
- Reporting
Specifically, compliance reviews and feedback on study documents ensure adherence to country requirements, which is crucial for successful trial execution. Utilizing project coordination tools, such as Gantt charts, is among the Best Practices for Managing Multi-Country Clinical Trials, as they can visualize timelines and milestones, facilitating better planning and execution.
Regular status meetings serve as vital checkpoints to implement best practices for managing multi-country clinical trials, ensuring alignment and addressing challenges promptly. Moreover, utilizing best practices for managing multi-country clinical trials through a centralized data management system enables real-time data sharing and monitoring, significantly reducing discrepancies and enhancing data integrity. Training local teams on standardized protocols fosters consistency and encourages effective collaboration across sites, particularly in areas like Barranquilla, Colombia, which is being positioned as a leading destination for research; this approach aligns with the Best Practices for Managing Multi-Country Clinical Trials through collaboration between bioaccess™ and Caribbean Health Group, supported by Colombia's Minister of Health.
As highlighted by the National Institutes of Health, investigators must feel valued and included in the study process; thus, adhering to the Best Practices for Managing Multi-Country Clinical Trials, providing regular feedback through structured check-ins and updates should be central to the communication strategy. Additionally, implementing the Best Practices for Managing Multi-Country Clinical Trials by creating a post-trial checklist ensures that critical tasks are not overlooked and that findings are documented accurately, which is vital for future reference and compliance. The function of technology in study management is further demonstrated by the use of electronic data capture systems, which enhance efficiency and data precision, thus aligning with the Best Practices for Managing Multi-Country Clinical Trials and contributing to the overall success of studies.
By applying the Best Practices for Managing Multi-Country Clinical Trials, research managers can improve the effectiveness and success of their global initiatives and positively influence regional economies through job creation and healthcare enhancement.
Addressing Cultural and Logistical Considerations in Global Trials
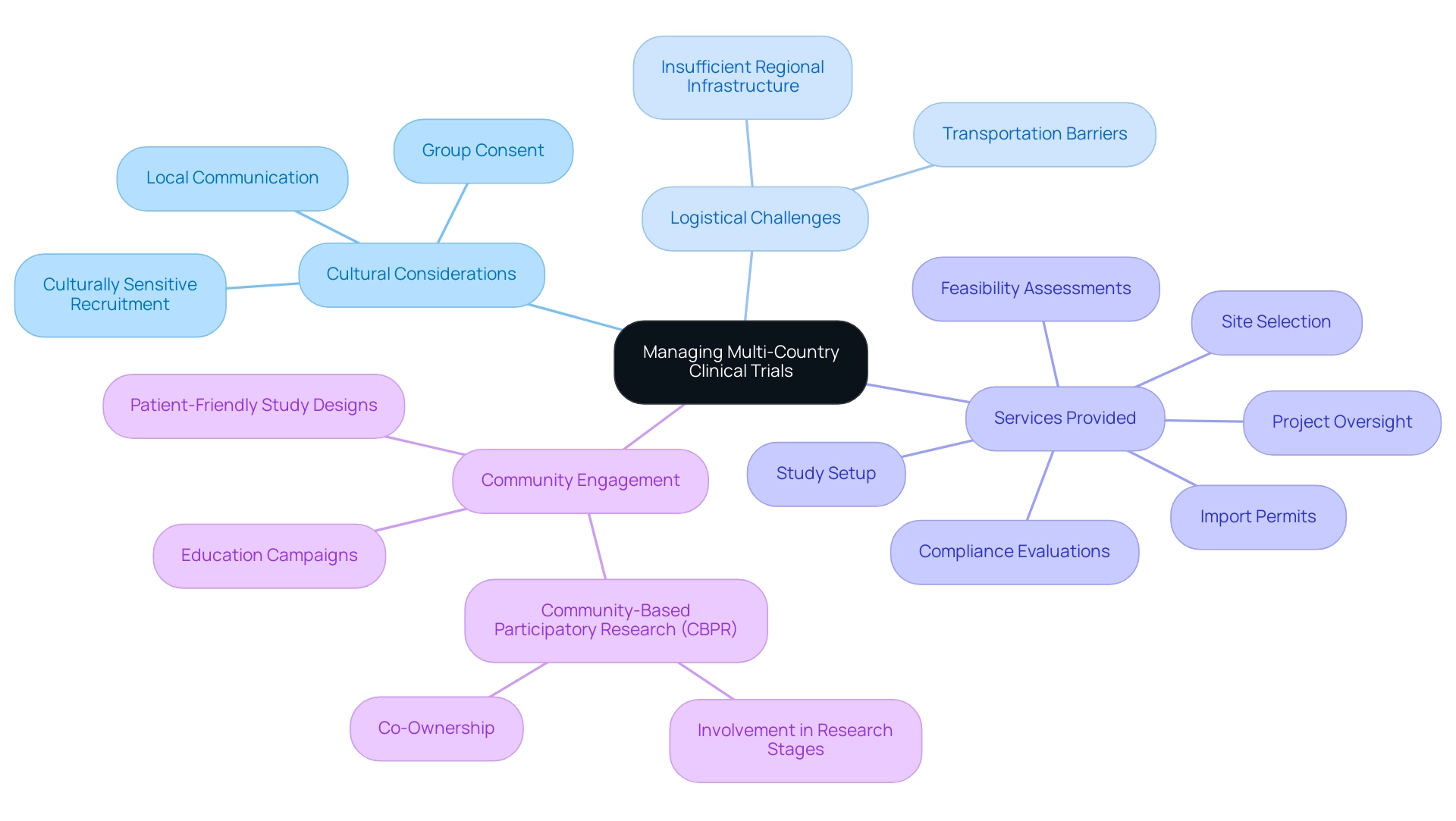
Understanding cultural nuances is essential as part of the best practices for managing multi-country clinical trials, significantly impacting participant recruitment and engagement in global research studies. For instance, certain cultures may favor group consent over individual consent, necessitating adaptations in the consent process to align with community values. Our extensive clinical study coordination services include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
All aimed at addressing logistical challenges such as transportation barriers and insufficient regional infrastructure that frequently obstruct access to study sites.
In addition, our services include detailed reporting on study status, inventory management, and tracking both serious and non-serious adverse events, ensuring compliance with country requirements and enhancing the overall integrity of the trials. This holistic approach not only facilitates recruitment but also positively affects community economies through job creation and healthcare enhancement. For example, the NHB cohort saw an increase in recruitment from 19.6% to 35.8%, illustrating the potential effectiveness of culturally sensitive recruitment approaches.
Hiring staff from the area who understand community dynamics can significantly enhance trust and improve recruitment rates. Adjusting communication materials to represent local languages and cultural practices is essential for encouraging greater participation and retention throughout the study. As mentioned by Anthony Haywood, Vice President of Clinical Trials Optimization at MEDiSTRAVA, 'In 2024, the trends in recruitment and retention in studies emphasize a more patient-centric, technologically advanced, and inclusive approach.'
This evolution highlights the urgent requirement for Best Practices for Managing Multi-Country Clinical Trials, particularly in addressing issues like participant fatigue and limited knowledge of research studies. Proposed solutions such as education campaigns and patient-friendly study designs can address these challenges effectively. Community-Based Participatory Research (CBPR) exemplifies an effective model by actively involving community members in all stages of the research process, ensuring that studies are not only relevant to community needs but also cultivate co-ownership, thereby leading to higher engagement and participation rates.
Leveraging Technology for Enhanced Clinical Trial Management
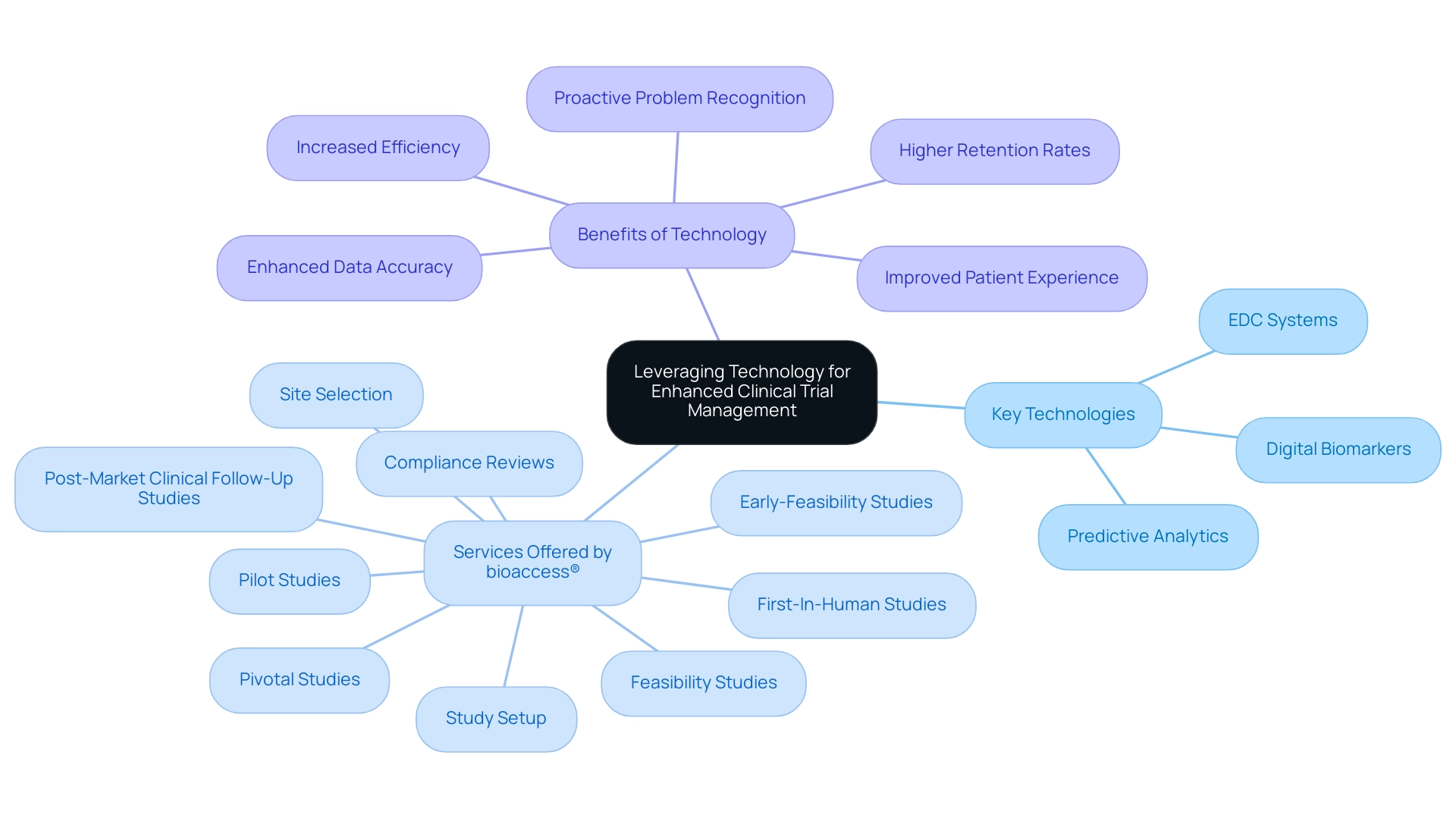
The incorporation of cutting-edge technology, especially AI and digital resources, is essential for enhancing the coordination of multi-country research studies, adhering to the Best Practices for Managing Multi-Country Clinical Trials to make them more patient-friendly and efficient. By implementing electronic data capture (EDC) systems, organizations can facilitate real-time data entry and monitoring, significantly reducing the errors commonly associated with traditional paper-based methods. This relates to extensive research management services, such as:
- Feasibility studies
- Compliance reviews
- Study setup
- Site selection
offered by skilled teams like bioaccess®, which has over 20 years in Medtech.
Recent statistics indicate that studies utilizing EDC systems report improved accuracy and efficiency in data collection processes. Furthermore, digital biomarkers—objective and quantifiable measures of health passively collected from wearables and other sensor signals—are becoming increasingly important in this space. Mobile applications designed for participant engagement not only enhance communication but also contribute to higher retention rates by streamlining follow-ups and providing participants with timely information.
As Chris Harrop pointed out, 'The incorporation of technology in medical studies is not merely about efficiency; it’s about improving the patient experience.' Utilizing virtual meetings and collaboration platforms is one of the best practices for managing multi-country clinical trials, as they play a crucial role in enabling seamless coordination between teams across different countries and fostering a culture of collaboration that transcends geographical barriers. Additionally, the use of predictive analytics acts as an essential instrument for recognizing potential problems before they escalate, enabling timely and proactive measures that keep processes on track.
A notable example is the use of prognostic tools, which leverage digital devices to predict a patient's future disease course by analyzing biometric and symptom data, providing insights into therapy response and risk of disease recurrence. As we progress into 2024, the newest technology trends in research management highlight the necessity for organizations to remain proactive by embracing innovative solutions that improve operational efficiency and patient involvement. These advancements significantly contribute to regional economies through job creation and healthcare improvement, particularly through the specific services offered by bioaccess® in managing:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies
The Importance of Local Collaboration in Multi-Country Clinical Trials
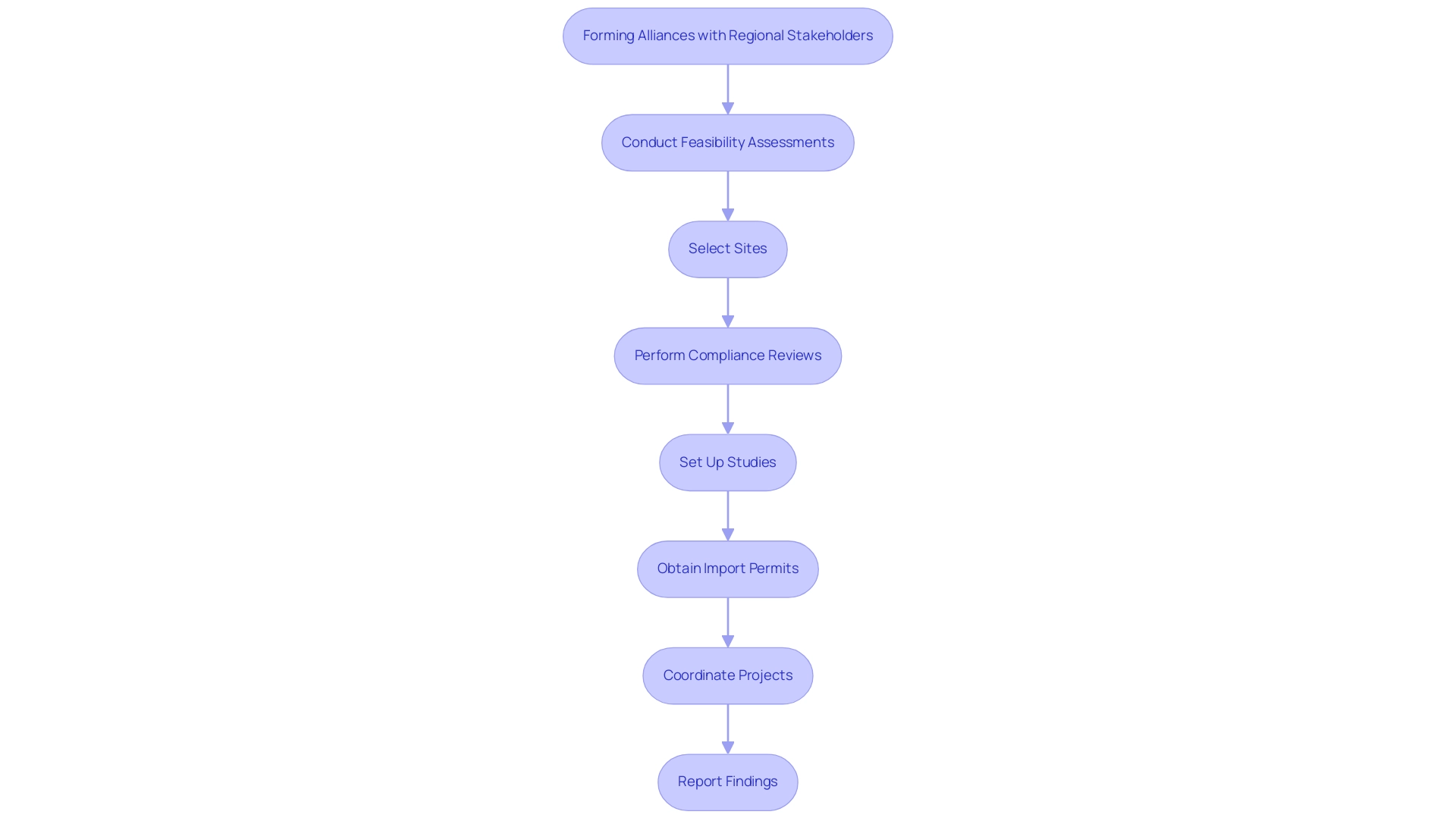
Forming strong alliances with regional stakeholders, including researchers, regulatory agencies, and ethics boards, is vital for adhering to the best practices for managing multi-country clinical trials. These collaborations not only ensure adherence to regional regulations but also yield invaluable insights into regional practices and patient preferences. Our advisory board plays a crucial role in guiding these partnerships, utilizing their expertise to improve the success of research studies while ensuring that our approach aligns with both international standards and regional nuances.
Moreover, our extensive research study oversight services include:
- Feasibility assessments
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project coordination
- Reporting
Our project management services include:
- Detailed planning
- Risk assessment
- Ongoing monitoring
This organized method is intended to enable seamless operations, especially under the supervision of INVIMA, Colombia's National Food and Drug Surveillance Institute, acknowledged as a Level 4 health authority by PAHO/WHO.
The influence of Medtech research studies goes beyond single experiments; they enhance regional economies through job creation, economic development, and healthcare advancements. For example, studies have indicated that clinical studies can result in substantial job prospects in research and healthcare fields, promoting regional economic growth. Ashley Moultrie, the Senior Director of Diversity, Equity, and Inclusion & Community Engagement at Javara, emphasizes the importance of fostering relationships:
We can only do that if we all are willing to sit at the table and have the conversation around the patient experience and making sure our patients have that, what we call, white glove service, and making sure that they want to come back and they want to participate.
This patient-focused approach not only boosts the study's credibility but also encourages community acceptance, resulting in better participant recruitment.
Ongoing communication and collaborative planning sessions with regional partners are essential for aligning objectives and methodologies, reflecting the best practices for managing multi-country clinical trials to create a unified strategy that promotes study success. Engaging with patient advocacy groups is instrumental in bolstering recruitment efforts by increasing awareness and trust within the community. This is evidenced by community-based participatory research conducted by Schroepfer in 2009, which successfully improved recruitment and retention through regional personnel involvement.
Given that 18.8% of studies were conducted remotely, this statistic underscores the challenges faced in recruitment and highlights the necessity of local collaboration for effective participation in clinical trials.
Conclusion
Conducting multi-country clinical trials requires a comprehensive understanding of diverse regulatory, ethical, and cultural landscapes. The article underscores the importance of navigating these complexities through effective project management, local expertise, and cultural sensitivity. By prioritizing thorough preparation—such as feasibility studies, compliance reviews, and stakeholder collaboration—researchers can enhance the integrity and efficiency of clinical trials.
The integration of advanced technologies, such as electronic data capture systems and digital biomarkers, plays a crucial role in streamlining processes and improving participant engagement. These innovations not only reduce errors but also foster a more patient-centric approach, ultimately leading to higher retention rates and successful outcomes. Additionally, establishing robust partnerships with local stakeholders ensures compliance with regulations and enhances the understanding of patient needs, which is vital for effective recruitment and community trust.
In summary, the success of multi-country clinical trials hinges on a multifaceted strategy that encompasses regulatory adherence, technological advancements, and cultural considerations. By leveraging local insights and fostering collaboration, organizations can navigate the complexities of global research effectively, contributing positively to local economies and healthcare systems. As the landscape of clinical trials continues to evolve, embracing these strategies will be essential for achieving impactful and ethical medical research outcomes.
Frequently Asked Questions
What are the key considerations for managing multi-country clinical trials?
Key considerations include understanding distinct regulatory and ethical frameworks by region, conducting feasibility studies, selecting research sites, identifying principal investigators, and ensuring compliance with local laws regarding informed consent and reporting obligations.
Why is it important to comply with local regulations in clinical trials?
Compliance with local regulations is essential to ensure the ethical conduct of research, protect participant rights, and avoid legal complications. It also ensures adherence to necessary approvals from ethics committees and health ministries.
What is the significance of the Clinical Trials Regulation (EU) No 536/2014 in the European Union?
This regulation establishes rigorous standards for study approval and reporting procedures, requiring a standard review process that may take up to 106 days for a complete application, highlighting the importance of regulatory compliance in clinical studies.
How can researchers effectively navigate complex regulations in different regions?
Engaging regional regulatory consultants with expertise in biomedical engineering and regulatory affairs can help researchers address ethical considerations and reduce the risk of delays or legal issues.
What role does ongoing training play in managing multi-country clinical trials?
Ongoing training for the research team on regulations ensures compliance throughout the process and fosters awareness of the evolving regulatory landscape, ultimately enhancing the integrity of the research.
What is the expedited transition procedure mentioned in the article?
The expedited transition procedure allows for a minimum dossier review in just 22 days, streamlining regulatory processes and improving efficiency.
What best practices should be followed to manage multi-country clinical trials effectively?
Best practices include clear communication channels, well-defined roles and responsibilities, conducting feasibility studies, compliance reviews, project oversight, and utilizing project coordination tools like Gantt charts.
How can technology enhance the management of clinical trials?
Utilizing electronic data capture systems improves efficiency and data precision, and a centralized data management system allows for real-time data sharing and monitoring, which enhances data integrity.
Why is it important to provide regular feedback to investigators during clinical trials?
Providing regular feedback through structured check-ins and updates ensures that investigators feel valued and included in the study process, which is crucial for maintaining motivation and engagement.
What is the impact of applying best practices in managing multi-country clinical trials?
Applying best practices can improve the effectiveness and success of global research initiatives, positively influencing regional economies through job creation and healthcare enhancement.

