Overview
This article examines best practices for medical device trial strategies in Mexico, underscoring the critical nature of regulatory compliance, effective recruitment, and collaboration with local stakeholders to achieve successful clinical studies. It illustrates how robust management services, such as those provided by bioaccess®, not only facilitate adherence to regulatory requirements but also enhance patient recruitment through community engagement. Furthermore, these services leverage local expertise to improve study outcomes, ultimately ensuring the safety and efficacy of medical devices in the market.
Introduction
In the dynamic landscape of medical device development, the significance of clinical trials in Mexico is paramount. These trials are crucial not only for ensuring regulatory compliance and patient safety but also for validating the effectiveness of innovative technologies poised to transform healthcare. As the demand for advanced medical solutions surges—particularly with a projected increase in the need for innovative technologies by 2025—the role of robust clinical trials becomes even more critical.
With Mexico accounting for a notable share of the global clinical trials market, the strategies employed in these trials carry far-reaching implications for manufacturers, patients, and the healthcare system at large. From navigating complex regulatory frameworks to overcoming recruitment challenges and establishing effective data management practices, the journey of bringing a medical device to market is intricate.
This article delves into the essential aspects of clinical trials in Mexico, highlighting best practices, stakeholder collaboration, and the pivotal role of organizations like bioaccess® in facilitating successful outcomes.
The Significance of Clinical Trials for Medical Devices in Mexico
Medical device trial strategies in Mexico are crucial for the effective launch of medical devices within the Mexican market, where regulatory adherence and patient safety are paramount. These evaluations not only confirm the safety and effectiveness of new devices but also yield essential information that informs regulatory decisions. As we approach 2025, the demand for innovative medical technologies in Mexico is on the rise, underscoring the necessity of robust assessments that ensure compliance with both local and global standards.
Recent insights reveal that in 2024, key evaluations emerged as the most significant revenue-generating segment within the research landscape, highlighting their vital role in market dynamics. This trend is expected to continue, with critical research indicating the fastest growth during the forecast period. Notably, Mexico accounted for 2.3% of the global medical device research market, emphasizing its status as a significant player in this arena.
Successful studies not only enhance the credibility of manufacturers but also facilitate market access, ultimately leading to improved patient care and public health outcomes in the region. As bioaccess® emphasizes, their comprehensive management services for research encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
All tailored to navigate the complexities of the regulatory landscape. With over 20 years of experience in Medtech, bioaccess® is exceptionally equipped to tackle the challenges faced by medical device startups, including regulatory obstacles and competitive pressures.
Moreover, bioaccess® utilizes a variety of tools for medical data management, enhancing precision and efficiency in data handling processes, which is critical for upholding regulatory compliance and ensuring patient safety.
The impact of Medtech research extends beyond individual studies, contributing to local economies through job creation and economic growth. As the landscape evolves, the importance of clinical studies in ensuring patient safety and regulatory compliance remains a cornerstone of medical device trial strategies in Mexico.
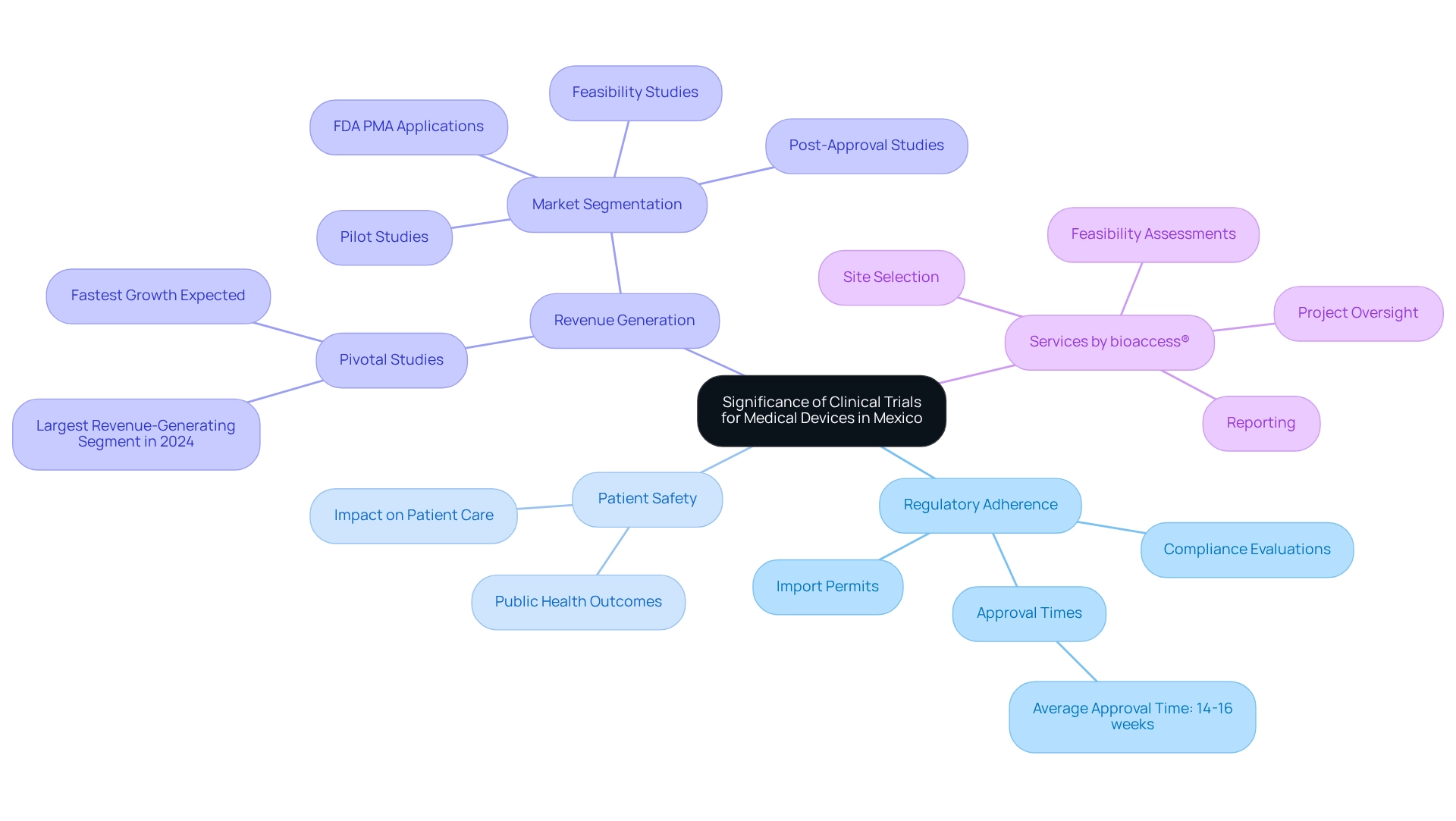
Navigating the Regulatory Framework for Medical Device Trials in Mexico
The regulatory environment for medical device studies in Mexico is primarily overseen by the Federal Commission for Protection against Sanitary Risks (COFEPRIS). Organizations aiming to conduct studies must navigate a complex framework that encompasses stringent requirements for ethical approvals and adherence to Good Clinical Practice (GCP). Engaging local regulatory experts, such as those from bioaccess®, is highly advisable, as they can provide invaluable insights into the submission process, anticipated timelines, and essential documentation necessary for compliance.
This process includes:
- Feasibility assessments
- Selection of research sites and principal investigators
- Thorough review and feedback on study documents to ensure alignment with national requirements
It is also crucial to recognize that in emergency situations, the ethics committee may waive the requirement for written voluntary consent from research participants, thereby introducing a layer of flexibility in urgent scenarios. Furthermore, at the conclusion of a research study, the lead investigator must account for the investigational product, while the sponsor is responsible for the storage of the IP and overseeing the withdrawal and disposal of any unused products. This highlights the critical responsibilities associated with study management, which bioaccess® adeptly handles through comprehensive research study management services, including the import permit and nationalization of investigational devices.
Moreover, it is essential to remain vigilant regarding regulatory changes, as COFEPRIS is actively enhancing its procedures to boost efficiency and ensure patient safety in research. For example, if COFEPRIS issues a deficiency letter, manufacturers should prepare for an additional six to eight months for resolution, underscoring the significance of thorough preparation and a deep understanding of the regulatory landscape. As Dr. Sarah Bosshard, Head of Clinical at Congenius, states, "Navigating the regulatory landscape requires not only compliance but also a proactive approach to understanding the evolving requirements."
By prioritizing these strategies and insights, organizations can effectively navigate the regulatory landscape and implement medical device trial strategies in Mexico for successful assessments. Additionally, examining comparative instances, such as Vietnam's comprehensive evaluation procedure for medical studies, can provide significant insights into ensuring that ethical and scientific standards are upheld. With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, companies can advance their medical device evaluations with confidence.
Reach out to bioaccess® to discover more about our research management services.
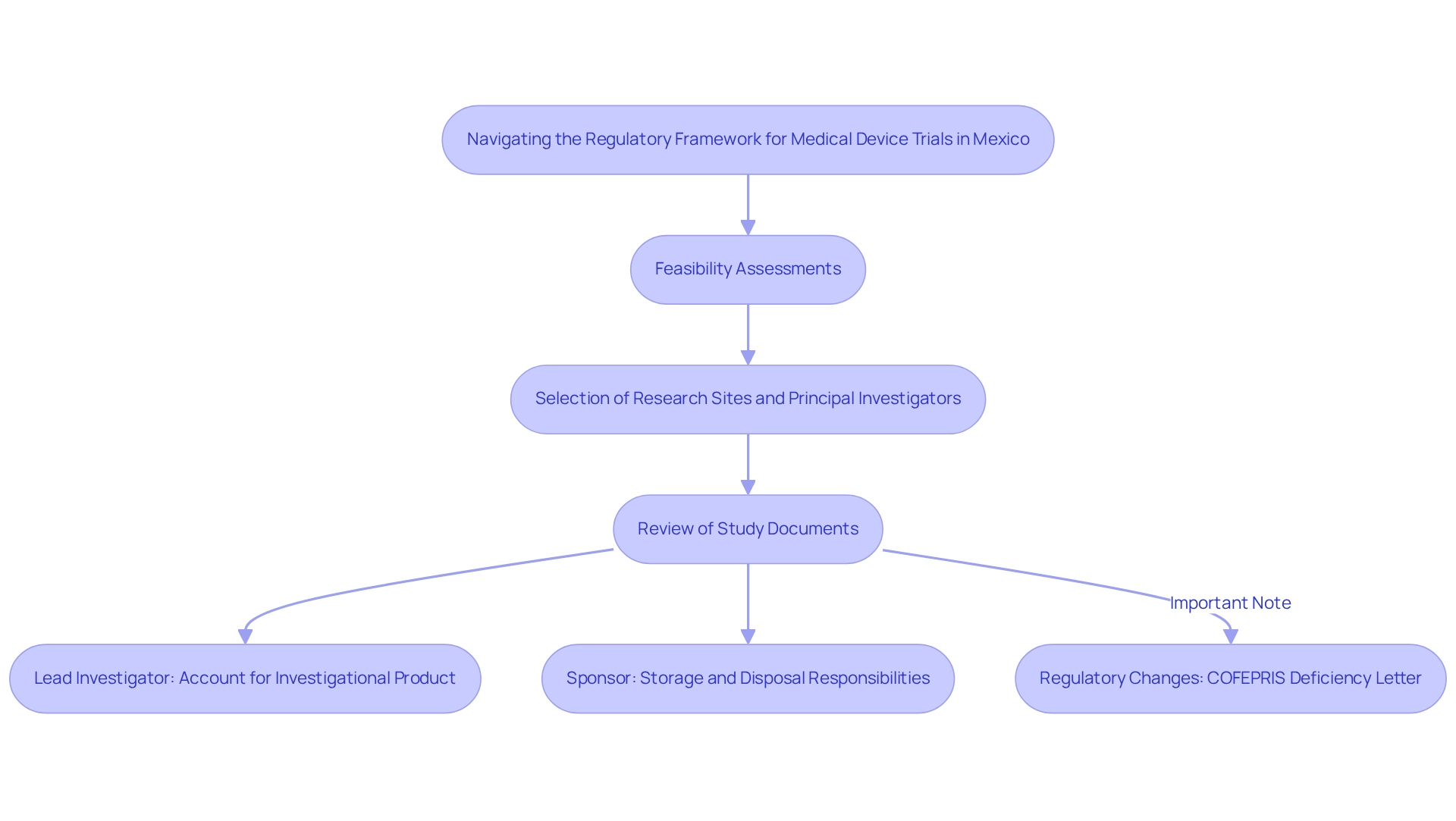
Strategies for Overcoming Recruitment Challenges in Mexican Clinical Trials
Recruitment challenges in clinical studies in Mexico can be effectively addressed through strategic medical device trials. A highly impactful approach involves leveraging local healthcare networks and community organizations, significantly enhancing outreach efforts. By actively involving healthcare providers and keeping them informed about ongoing studies, sponsors can encourage valuable referrals, capitalizing on established patient-physician relationships prevalent in the region.
Digital platforms and social media are crucial in broadening the reach to potential participants. These tools facilitate rapid and effective dissemination of information, particularly among younger demographics who are more engaged online. However, it is essential that recruitment materials are culturally sensitive and available in both Spanish and English to maximize engagement across diverse populations.
Building trust with potential participants stands as a key factor in improving recruitment rates. Clear communication regarding the study's purpose, procedures, and potential benefits can alleviate concerns and promote participation. Notably, a recent case study highlighted that 85% of patients were unaware of their options for involvement in research studies at the time of diagnosis, revealing a substantial opportunity for outreach.
Moreover, 75% of those surveyed indicated a willingness to enroll if informed earlier, underscoring the importance of proactive engagement strategies.
The Mexican clinical research landscape is evolving, bolstered by significant investments in healthcare infrastructure, including a recent $700 million expansion by one of Mexico’s largest hospital chains, resulting in 15 new facilities. This growth not only enhances the capacity to conduct studies but also provides a broader network for patient recruitment, enabling sponsors to engage a wider patient base. As Sergio A. Sanchez, a former research consultant at Deallus Consulting, aptly noted, "Sponsors need to thoroughly assess the partner’s performance in selecting study sites and investigators, enrolling subjects, and meeting study deadlines."
Additionally, the substantial pediatric population in Latin America presents an expanding opportunity for recruitment, as this demographic increasingly engages in research studies. By employing these strategies and nurturing robust local collaborations, study sponsors can navigate the unique challenges of recruitment in Mexico and enhance overall study success through the implementation of medical device trial strategies. Furthermore, the partnership between bioaccess™ and Caribbean Health Group to establish Barranquilla as a prominent location for medical studies, supported by Colombia's Minister of Health, underscores the region's potential and fosters a competitive atmosphere that can benefit Mexican studies.
bioaccess™ provides comprehensive management services for research, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. This partnership has achieved a reported 50% decrease in recruitment duration and 95% retention rates, demonstrating bioaccess™'s efficiency in improving research processes. Furthermore, the economic impact of Medtech research studies, such as job creation and healthcare enhancement, further highlights the significance of these initiatives in the region.
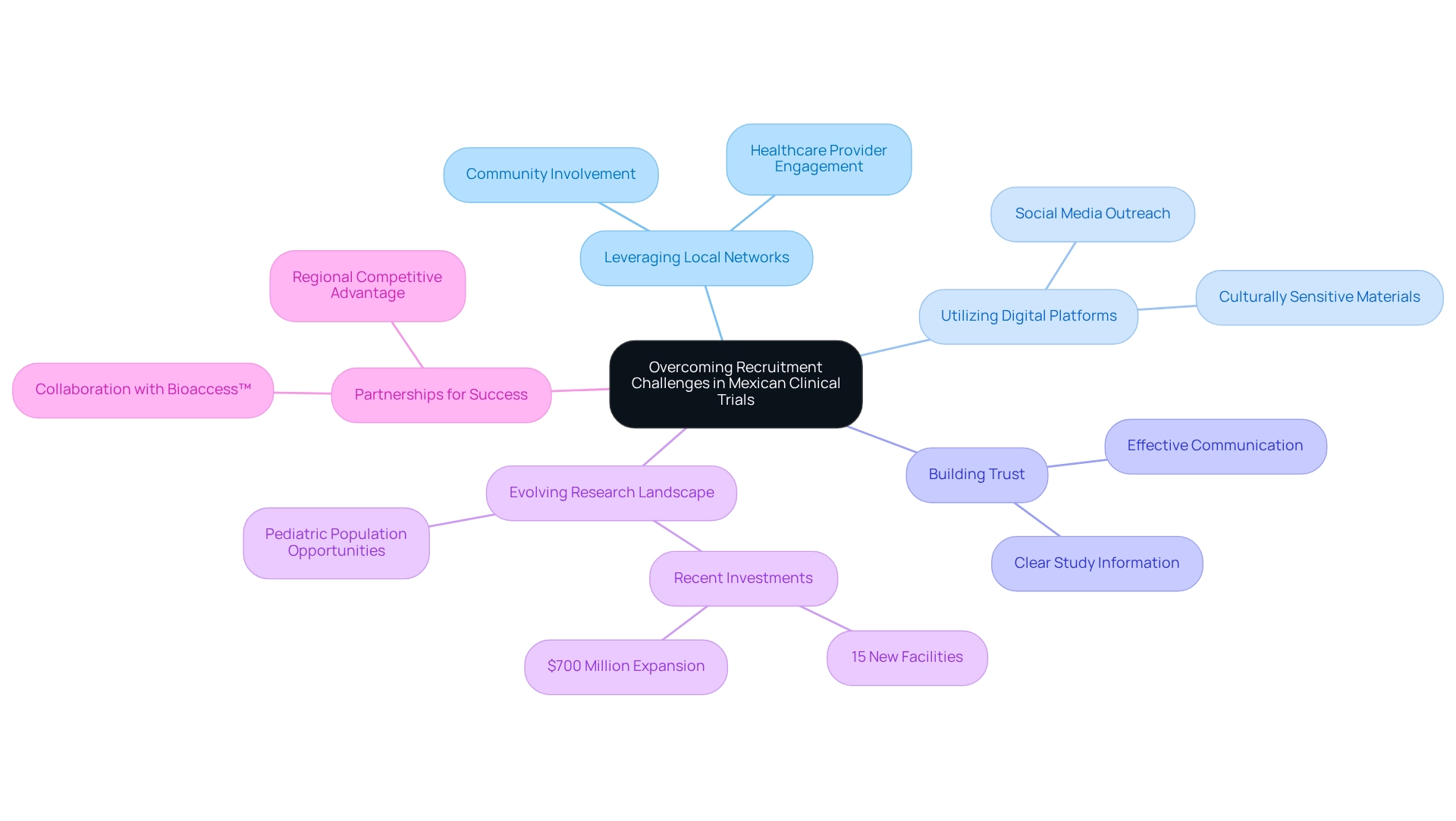
Key Considerations for Selecting Clinical Trial Sites in Mexico
Choosing clinical research locations in Mexico requires a thorough evaluation of various crucial factors, particularly those pertinent to medical device trial strategies. Foremost among these is the site's infrastructure, which must be equipped with the essential medical apparatus to meet the study's requirements. Equally vital are the qualifications and experience of the site personnel, especially the principal investigators, as their expertise directly impacts the success of the study.
Another critical factor is the site's patient population, which should align with the study's eligibility criteria. Proximity to urban centers can significantly enhance recruitment efforts, as these areas typically have a larger pool of potential participants. However, rural locations can offer unique patient demographics that may be beneficial for specific studies, providing access to groups that are often underrepresented in clinical research.
For instance, statistics indicate that Mexican-American women are three times more likely to use herbs than the overall population, guiding recruitment strategies for studies focused on herbal treatments.
Moreover, fostering a strong relationship with site staff is essential. Their commitment to the study can lead to enhanced collaboration and ultimately better results. As noted by Gotuzzo from Universidad Peruana Cayetano Heredia, "These challenges demand a strategic approach to recruitment," emphasizing the importance of effectively addressing recruitment hurdles.
Furthermore, the Clinical Trial Readiness Score has been developed to rank countries by study type, identifying investment opportunities for underutilized nations like Mexico. This score underscores Mexico's potential as a favorable site for medical device trial strategies, making it imperative for researchers to consider when selecting locations.
In this context, bioaccess® emerges as a leading Contract Research Organization (CRO) that supports medical device trial strategies in Mexico and throughout Latin America. With over 20 years of experience, bioaccess® offers comprehensive management services for research projects, including feasibility assessments, site selection, compliance reviews, project setup, import permits, project oversight, and reporting. Their focus on Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies ensures that researchers can effectively navigate the complexities of the Latin American Medtech landscape, particularly in relation to medical device trial strategies in Mexico.
Insights from the case analysis titled 'Cost-Competitive CRO for Small Biotech' further illustrate how budget-friendly CRO services can significantly assist small biotech companies in conducting research effectively and efficiently. By prioritizing these factors—site infrastructure, staff qualifications, patient demographics, and interpersonal relationships—researchers can enhance their study site selection process in Mexico, paving the way for successful study execution while contributing to local economic growth and healthcare improvement.
Best Practices for Data Management and Analysis in Clinical Trials
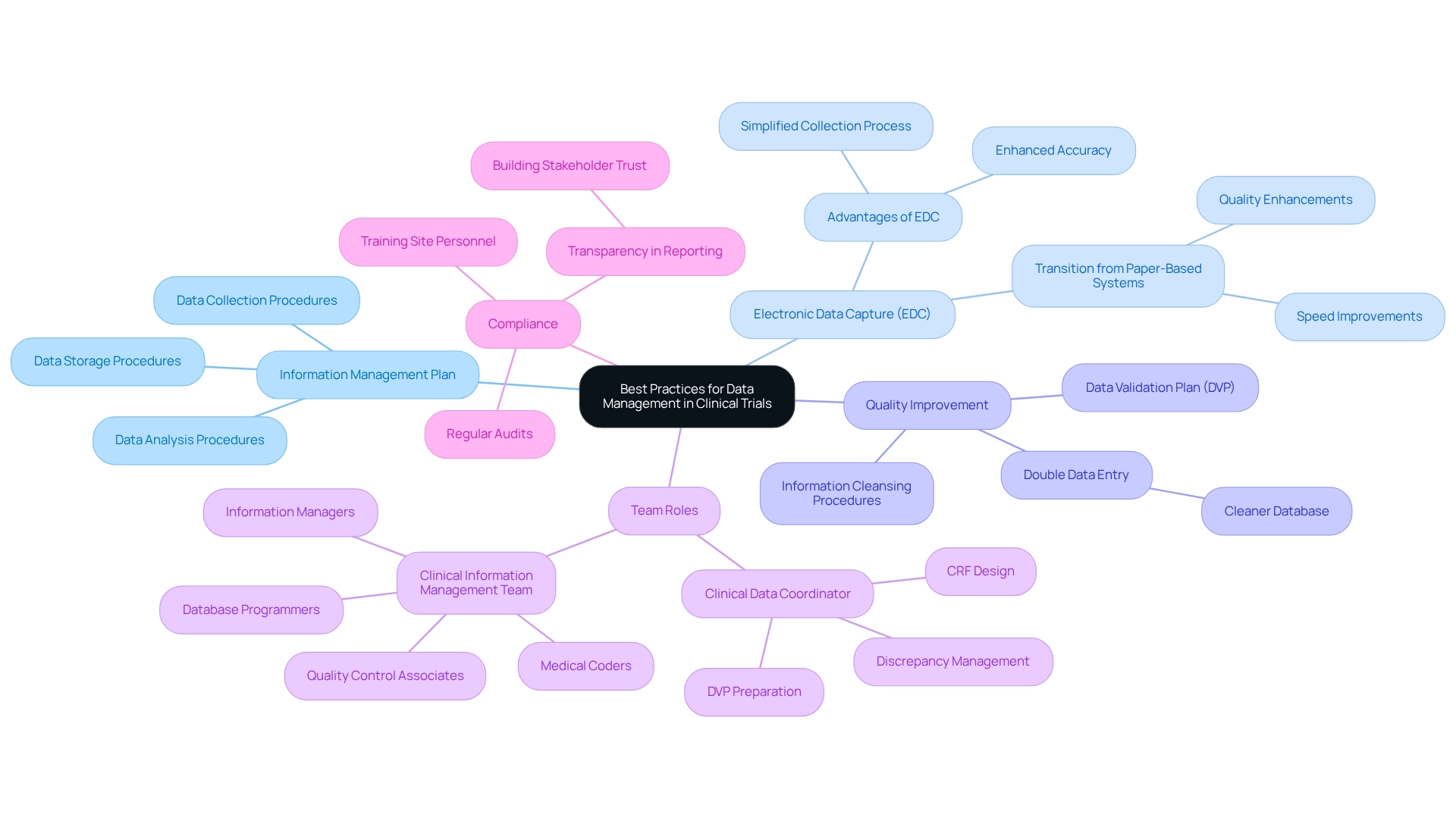
Optimal methods for information management in clinical trials are crucial for guaranteeing the integrity and reliability of trial outcomes. Establishing a comprehensive information management plan is essential, detailing procedures for collection, storage, and analysis. The adoption of electronic information capture (EDC) systems offers significant advantages, as these systems greatly enhance accuracy and simplify the collection process.
Research indicates that employing double information input can yield a clearer database compared to single information input, thereby enhancing information quality. At bioaccess, we provide vital services, including feasibility analysis and the selection of research locations and principal investigators (PIs), which are fundamental in establishing a robust foundation for information management. Our offerings also encompass detailed reviews and feedback on study documents to ensure adherence to country requirements, complemented by extensive setup procedures that facilitate seamless project initiation. The Clinical Data Coordinator plays a pivotal role in this process, overseeing critical tasks such as Case Report Form (CRF) design, Data Validation Plan (DVP) preparation, and discrepancy management.
Moreover, the Clinical Information Management (CIM) team comprises various positions, including Information Managers, Database Programmers, Medical Coders, and Quality Control Associates, each contributing to efficient information management in clinical trials. Regular audits and monitoring are essential to ensure compliance with management protocols, while training site personnel on entry procedures and the significance of information integrity is vital for minimizing errors. A clearly defined procedure for information cleansing and validation before analysis will enhance the dependability of trial outcomes. Furthermore, ensuring transparency in reporting is critical for fostering trust with stakeholders and regulatory organizations.
For instance, the transition from paper-based to electronic information management systems has demonstrated improvements in both the speed and quality of information generation, addressing the pharmaceutical sector's demand for more efficient drug development processes. According to the Pharmaceutical R&D Efficiency Index, pharmaceutical firms that implement end-to-end information management solutions reduce their drug development cycle by an average of 1.5 years and increase their chances of regulatory approval by 23%. By adhering to these best practices and leveraging comprehensive trial management services, including robust reporting mechanisms, trials can achieve elevated standards of information management, ultimately leading to more successful outcomes and contributing to global health enhancement.
The Role of Post-Market Clinical Follow-Up Studies in Device Evaluation
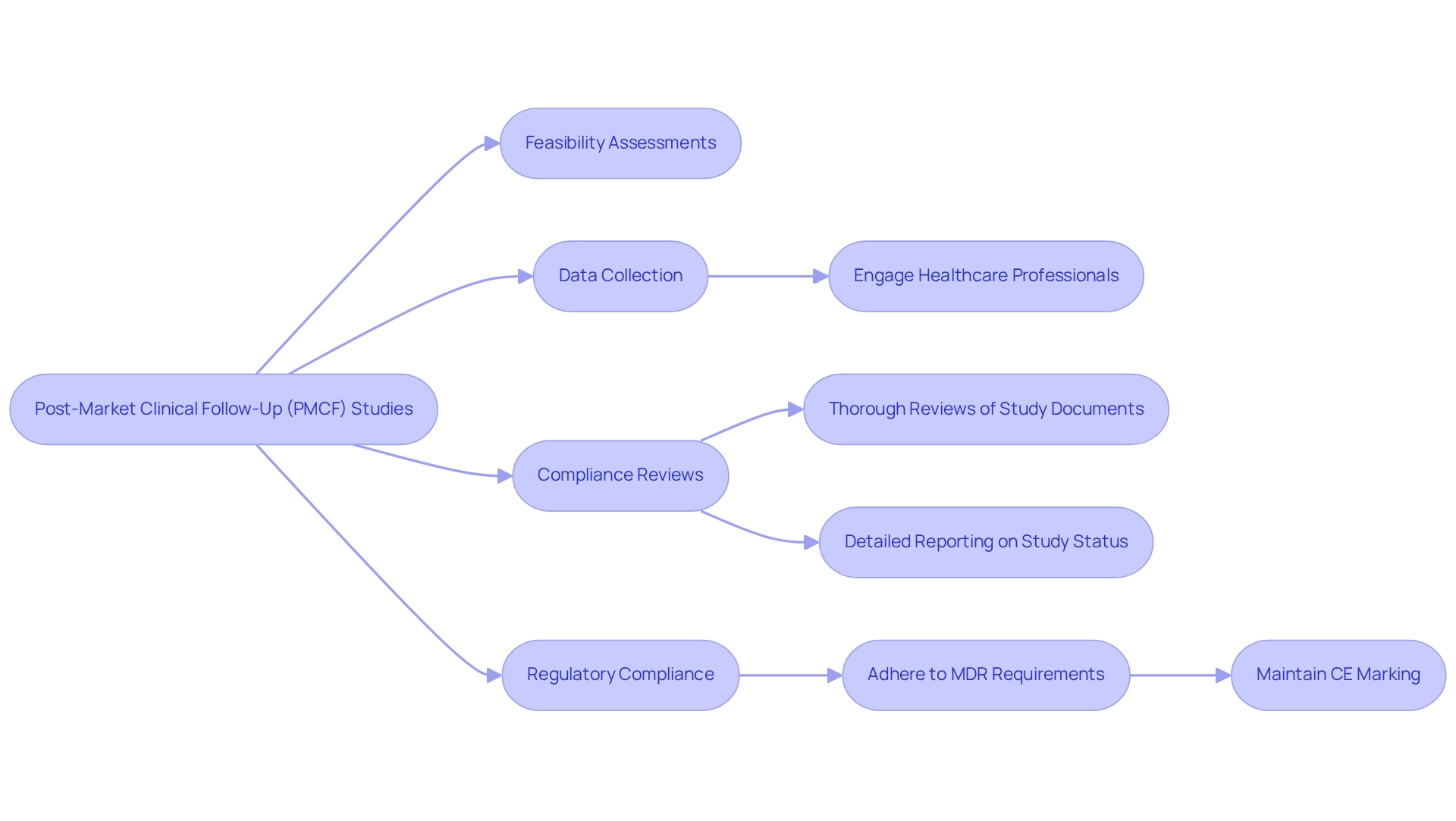
Post-market clinical follow-up (PMCF) investigations are crucial for gathering real-world data regarding the safety and performance of medical devices after they have entered the market. These analyses are essential in identifying potential issues that may arise post-launch, providing manufacturers with vital insights to enhance their products. As Jasper, a MedTech industry expert with 20 years of experience, states, "PMCF evaluations are essential for understanding how devices perform in real-world settings, which is crucial for ongoing innovation and safety."
At bioaccess®, we offer a comprehensive approach to PMCF research, ensuring that each plan is meticulously structured with clear objectives, methodologies, and timelines to effectively guide the process. Our services encompass:
- Feasibility assessments and selection of research sites
- Thorough reviews and feedback on study documents to ensure compliance with country regulations
- Detailed reporting on study status and adverse events
Engaging healthcare professionals and patients throughout the PMCF process significantly enhances data collection, ensuring that findings are relevant to real-world scenarios. This collaborative approach not only enhances the relevance of the data but also fosters trust and transparency between manufacturers and the healthcare community.
Moreover, compliance with regulatory requirements for PMCF is essential for maintaining market authorization and safeguarding patient safety. The Medical Device Regulation (MDR), introduced in 2017, stresses the importance of post-market surveillance for medical devices in the EU market. Adhering to the MDR's post-market monitoring requirements is vital for manufacturers to secure and retain the CE marking for their devices.
In Latin America, where regulatory landscapes can differ, understanding and adhering to medical device trial strategies in Mexico is crucial for successful device evaluation and market presence. By prioritizing PMCF research, manufacturers can not only fulfill regulatory obligations but also contribute to the continuous improvement of medical device safety and efficacy, ultimately benefiting patients and healthcare systems alike. Jasper Van de Sande's extensive experience in market opportunity evaluations and real-world evidence analysis further underscores the significance of PMCF examinations in advancing medical device innovations.
To learn more about how bioaccess® can support you with your PMCF studies and other research services, SCHEDULE A MEETING today.
Collaborating with Local Stakeholders for Successful Clinical Trials
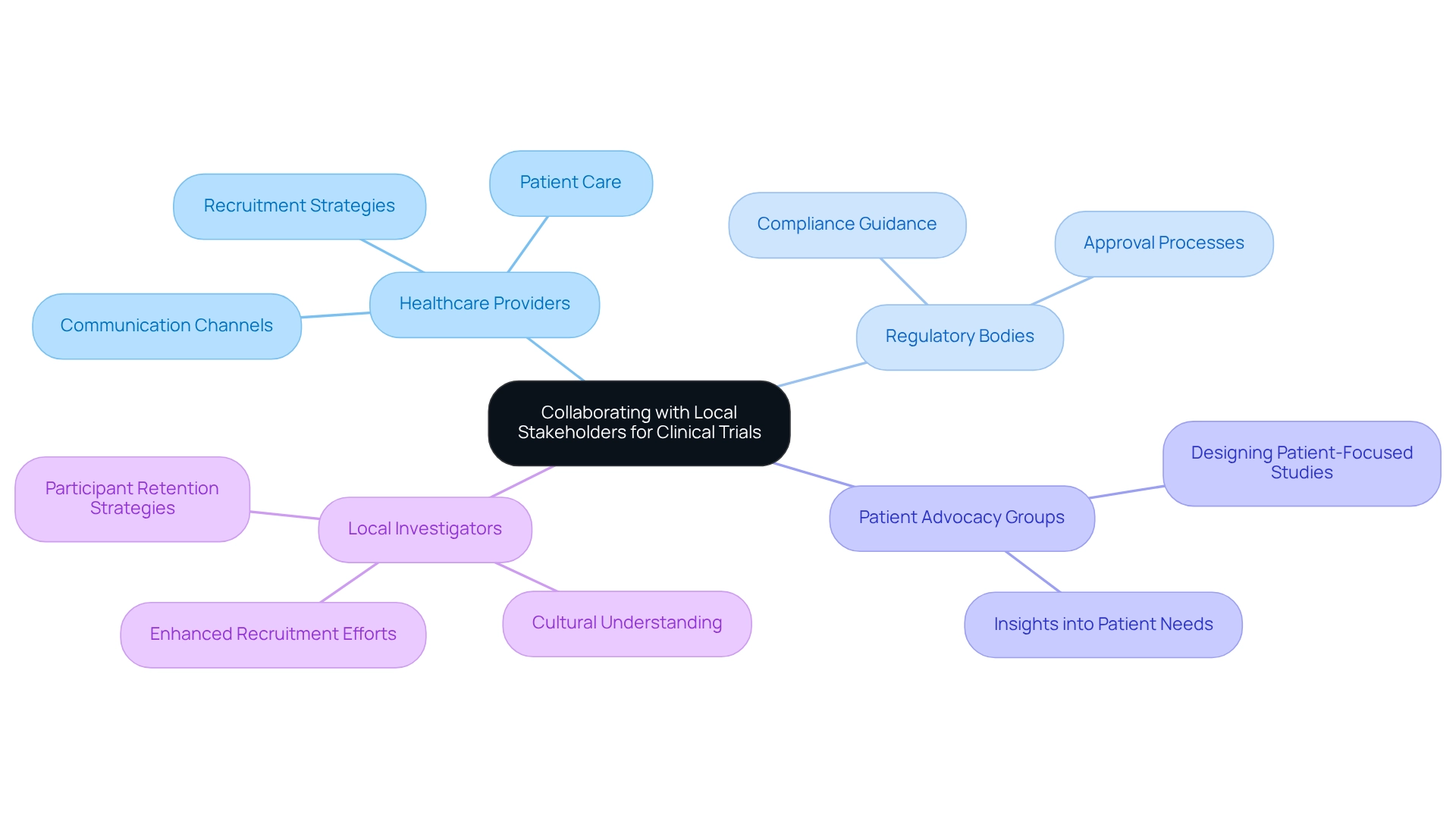
Effective collaboration with local stakeholders—including healthcare providers, regulatory bodies, and patient advocacy groups—is essential for the success of clinical studies in Mexico. Establishing clear communication channels and nurturing relationships based on trust can significantly improve execution. Engaging local investigators who possess a deep understanding of the cultural and healthcare landscape not only enhances recruitment efforts but also boosts participant retention rates.
Research indicates that studies conducted by local investigators achieve success rates significantly enhanced by their local expertise and relationships. Furthermore, including patient advocacy groups is crucial, as they provide valuable insights into patient needs and preferences, ensuring that studies are designed with the end-user in mind. This engagement can lead to more relevant and patient-focused study designs, which are increasingly vital in today’s research environment.
Consistently updating stakeholders about study progress and results not only strengthens these partnerships but also fosters a collaborative atmosphere that can drive innovation and efficiency in medical research.
In Mexico, the research landscape has seen notable growth, with a substantial increase in collaborations among local stakeholders. A recent survey revealed that over 70% of research sponsors reported successful partnerships with healthcare providers, underscoring the significance of these connections in navigating the region's complexities. As the Colombian government actively seeks to attract more research studies as part of its strategy to evolve into a knowledge economy by 2031, Mexico stands out as a key contributor in the Latin American market.
The obesity medication sector is projected to exceed $100 billion, underscoring the economic potential of successful studies in the region. With its bilingual, board-certified doctors and reduced research costs, Mexico presents an attractive environment for medical studies. By prioritizing teamwork and information exchange, stakeholders can ensure the successful advancement of medical device evaluations in Mexico, bolstered by medical device trial strategies and comprehensive research management services from specialists like bioaccess®, who focus on Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies.
Moreover, the collaboration between bioaccess® and Caribbean Health Group positions Barranquilla as a premier destination for clinical trials, further enhancing the region's appeal for clinical research.
Conclusion
The landscape of clinical trials in Mexico plays a crucial role in the successful development and introduction of medical devices. These trials are essential for ensuring regulatory compliance and patient safety, as well as for validating the efficacy of innovative technologies that have the potential to transform healthcare. Given Mexico's significant share in the global clinical trials market, the strategies employed in these trials carry substantial implications for manufacturers, healthcare providers, and patients alike.
Navigating the intricate regulatory framework, addressing recruitment challenges, and selecting appropriate trial sites are critical components that significantly influence the success of clinical trials. Collaborating with local stakeholders, including healthcare professionals and patient advocacy groups, enhances trial execution and promotes a patient-centered approach. Furthermore, robust data management practices are vital, as they ensure the integrity of trial outcomes and facilitate informed decision-making in the regulatory process.
Organizations like bioaccess® play a pivotal role in facilitating these complex processes, offering expertise and comprehensive clinical trial management services. As the demand for innovative medical technologies continues to rise, the significance of well-structured and effectively managed clinical trials in Mexico will only increase. By prioritizing best practices and fostering collaboration among stakeholders, the medical device industry can continue to thrive, ultimately benefiting public health and advancing healthcare solutions in the region.
Frequently Asked Questions
Why are medical device trial strategies important in Mexico?
Medical device trial strategies are crucial in Mexico for ensuring regulatory adherence and patient safety, confirming the safety and effectiveness of new devices, and providing essential information for regulatory decisions.
What is the role of bioaccess® in medical device trials?
Bioaccess® offers comprehensive management services for medical device research, including feasibility assessments, site selection, compliance evaluations, project oversight, and reporting, which help navigate the regulatory landscape.
What are the key components of the regulatory environment for medical device studies in Mexico?
The regulatory environment is overseen by COFEPRIS and includes stringent requirements for ethical approvals, adherence to Good Clinical Practice (GCP), and the necessity of engaging local regulatory experts for guidance.
What happens in emergency situations regarding participant consent in research studies?
In emergency situations, the ethics committee may waive the requirement for written voluntary consent from research participants, allowing for flexibility in urgent scenarios.
What responsibilities do lead investigators and sponsors have at the conclusion of a research study?
The lead investigator must account for the investigational product, while the sponsor is responsible for the storage, withdrawal, and disposal of any unused products.
How does COFEPRIS enhance its procedures for medical device studies?
COFEPRIS is actively enhancing its procedures to boost efficiency and ensure patient safety, which may include issuing deficiency letters that can extend the resolution process by an additional six to eight months.
What types of studies does bioaccess® manage?
Bioaccess® manages Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, helping companies advance their medical device evaluations confidently.
How does the medical device research landscape in Mexico impact the economy?
Medtech research contributes to local economies through job creation and economic growth, highlighting the broader impact of clinical studies beyond individual research.




