Introduction
The landscape of medical device trials is increasingly complex, necessitating a strategic approach to ensure successful outcomes. As the industry evolves, key design considerations emerge, from identifying relevant endpoints to ensuring regulatory compliance. This article delves into the critical aspects of medical device trial design, highlighting the importance of:
- Leveraging technology and data
- Fostering collaboration among stakeholders
- Implementing adaptive trial methodologies
By examining best practices and innovative strategies, it aims to equip researchers and organizations with the knowledge to navigate the multifaceted challenges of clinical trials, ultimately enhancing the safety and efficacy of medical devices in diverse patient populations.
Key Design Considerations for Medical Device Trials
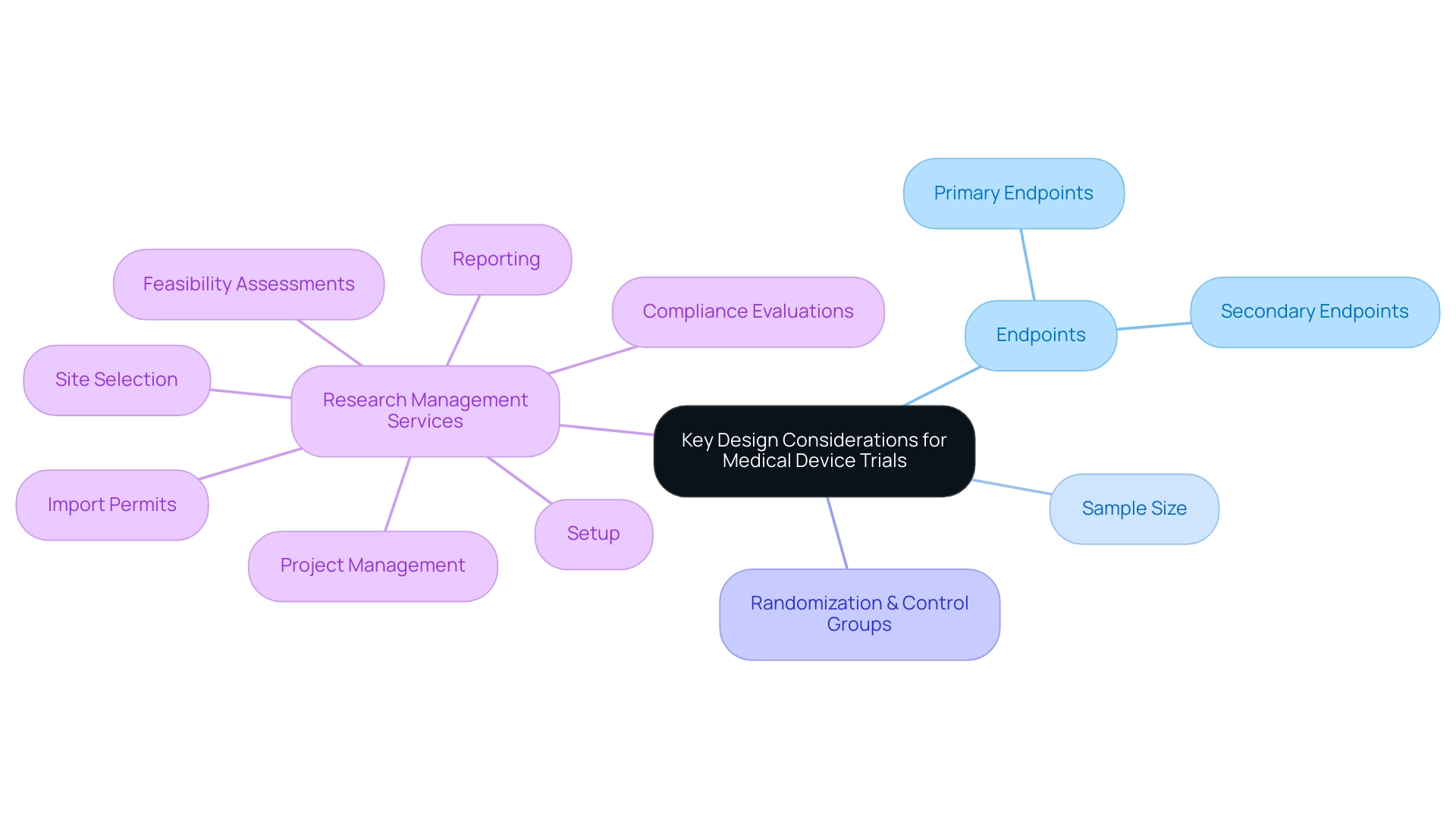
When creating medical device evaluations, several key factors must be considered for optimizing medical device trial design to ensure the project's effectiveness and integrity. First, optimizing medical device trial design requires:
- Identifying relevant primary and secondary endpoints that must align with the product’s intended use and regulatory expectations.
- Determining the appropriate sample size to ensure that the research is adequately powered to detect meaningful differences.
Randomization and control groups are critical components in optimizing medical device trial design, as these strategies help to mitigate biases and enhance the validity of the results. Moreover, optimizing medical device trial design by utilizing extensive research management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Reporting
can greatly simplify the process. With more than 20 years of experience in Medtech, bioaccess® provides a tailored strategy for overseeing research studies, guaranteeing flexibility and adaptability to address the distinct challenges of medical equipment investigations.
Experts such as Katherine Ruiz, a regulatory affairs specialist in Colombia, play an essential role in optimizing medical device trial design, enhancing recruitment, ensuring compliance with regulatory standards, and ultimately crafting strong study designs that produce significant data for regulatory submissions and healthcare applications.
Leveraging Technology and Data for Enhanced Trial Design
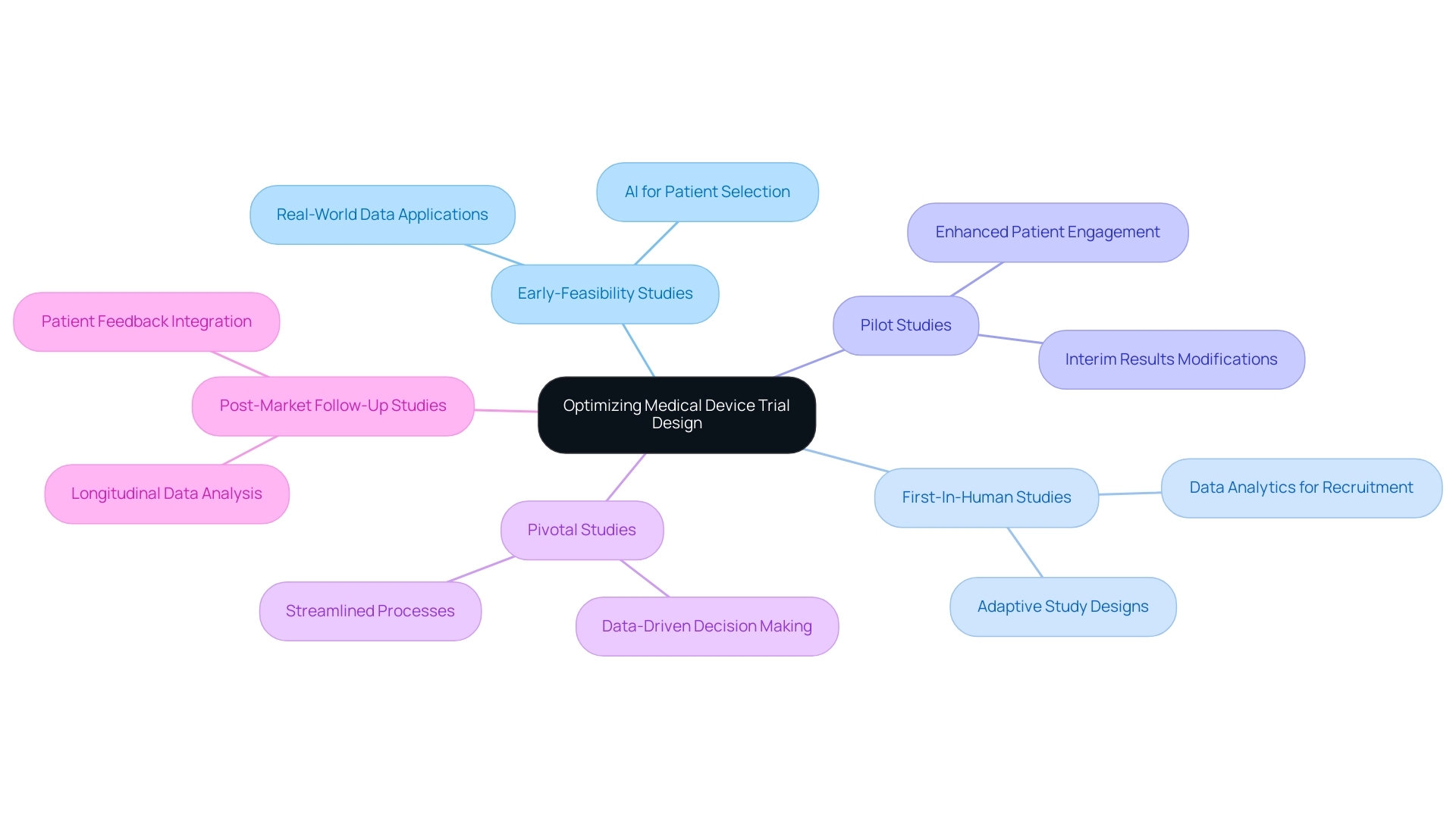
In the contemporary environment of medical research, optimizing medical device trial design by utilizing technology and data is essential for enhancing study outcomes. With over 20 years of experience in Medtech, bioaccess® leads the charge in Latin America, ensuring streamlined processes and successful outcomes through their expertise in conducting:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
The application of artificial intelligence (AI) enhances patient selection by identifying suitable candidates from large datasets, thus accelerating recruitment and improving the chances of success.
For example, GlobalCare Clinical Studies’ partnership with bioaccess™ led to more than a 50% decrease in recruitment time and an impressive 95% retention rate in studies in Colombia. Additionally, incorporating real-world data (RWD) allows researchers to gain insights from existing patient populations, leading to more relevant and applicable results. Furthermore, advanced data analytics can facilitate adaptive study designs, enabling modifications to the research based on interim results.
By adopting these innovative methods, alongside the tailored strategies bioaccess® utilizes to navigate clinical studies, researchers can significantly enhance their efforts in optimizing medical device trial design to improve the efficiency and results of medical assessments. Under the visionary leadership of Clinical Trial Manager Dr. Sergio Alvarado, this approach paves the way for faster and more effective product development.
Implementing Rigorous Regulatory Compliance Practices
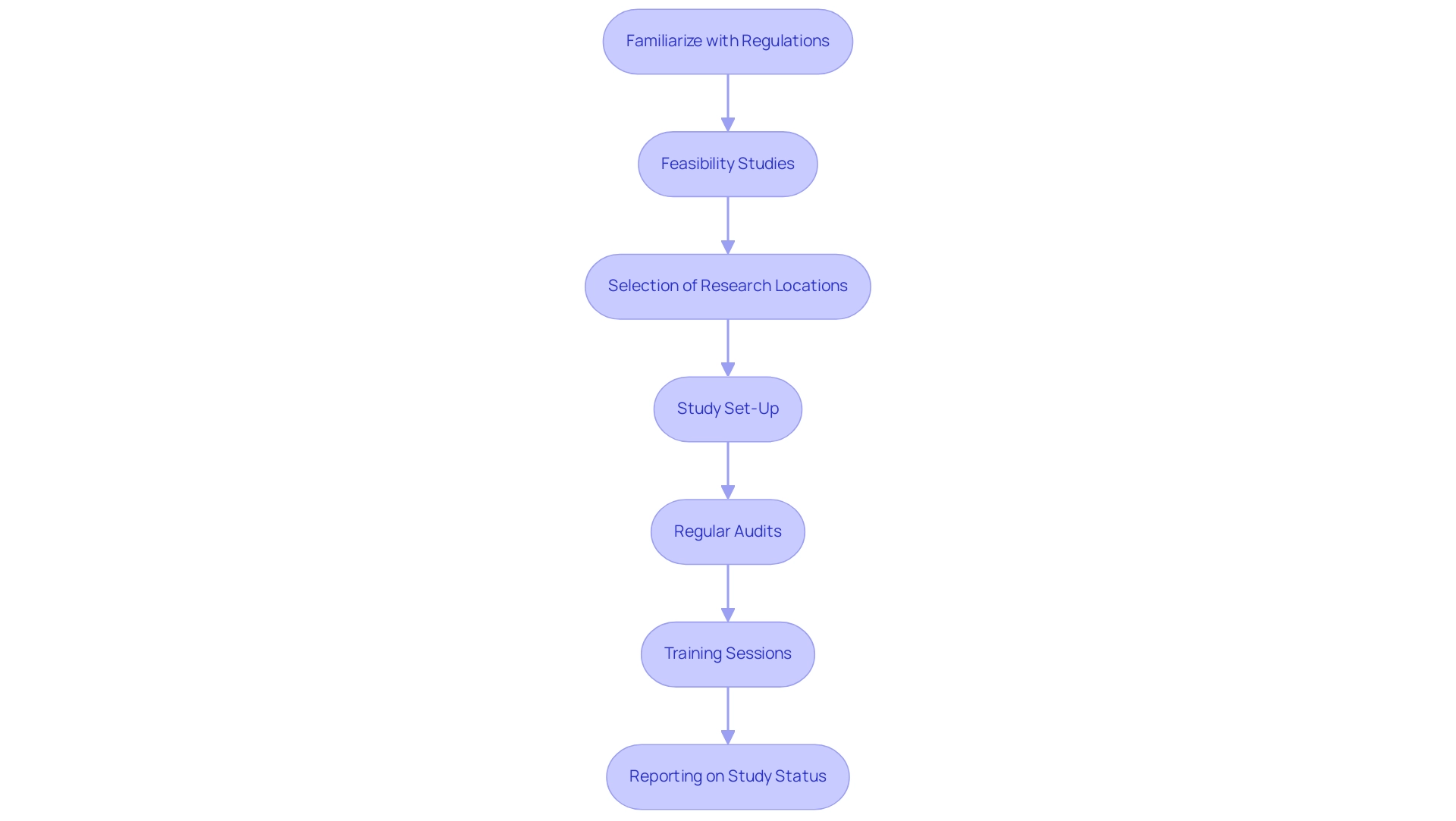
Regulatory compliance is a cornerstone of successful medical device studies, especially in the complex landscape of Latin America. It is essential to familiarize oneself with the relevant regulations set forth by bodies such as the FDA and EMA, including critical requirements for Investigational Device Exemptions (IDE) and the significance of Good Clinical Practice (GCP) guidelines. Startups frequently encounter regulatory obstacles, competition from established entities, and hiring challenges, making it essential to create a strong framework for monitoring compliance with these regulations throughout the process.
Our services encompass:
- Feasibility and selection of research locations and primary investigators
- Study set-up
- Thorough reporting on study status and adverse events
The expertise of seasoned professionals like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, a Regulatory Affairs expert in medical devices, can greatly enhance this framework. Their roles include ensuring adherence to Regulatory standards and offering guidance on management processes.
Regular audits and training sessions for the research team can help maintain high standards of compliance, ultimately protecting patient safety and enhancing the credibility of research results. Through comprehensive clinical study management services, including feasibility studies and project management, researchers can navigate these challenges effectively.
Fostering Collaboration Among Stakeholders
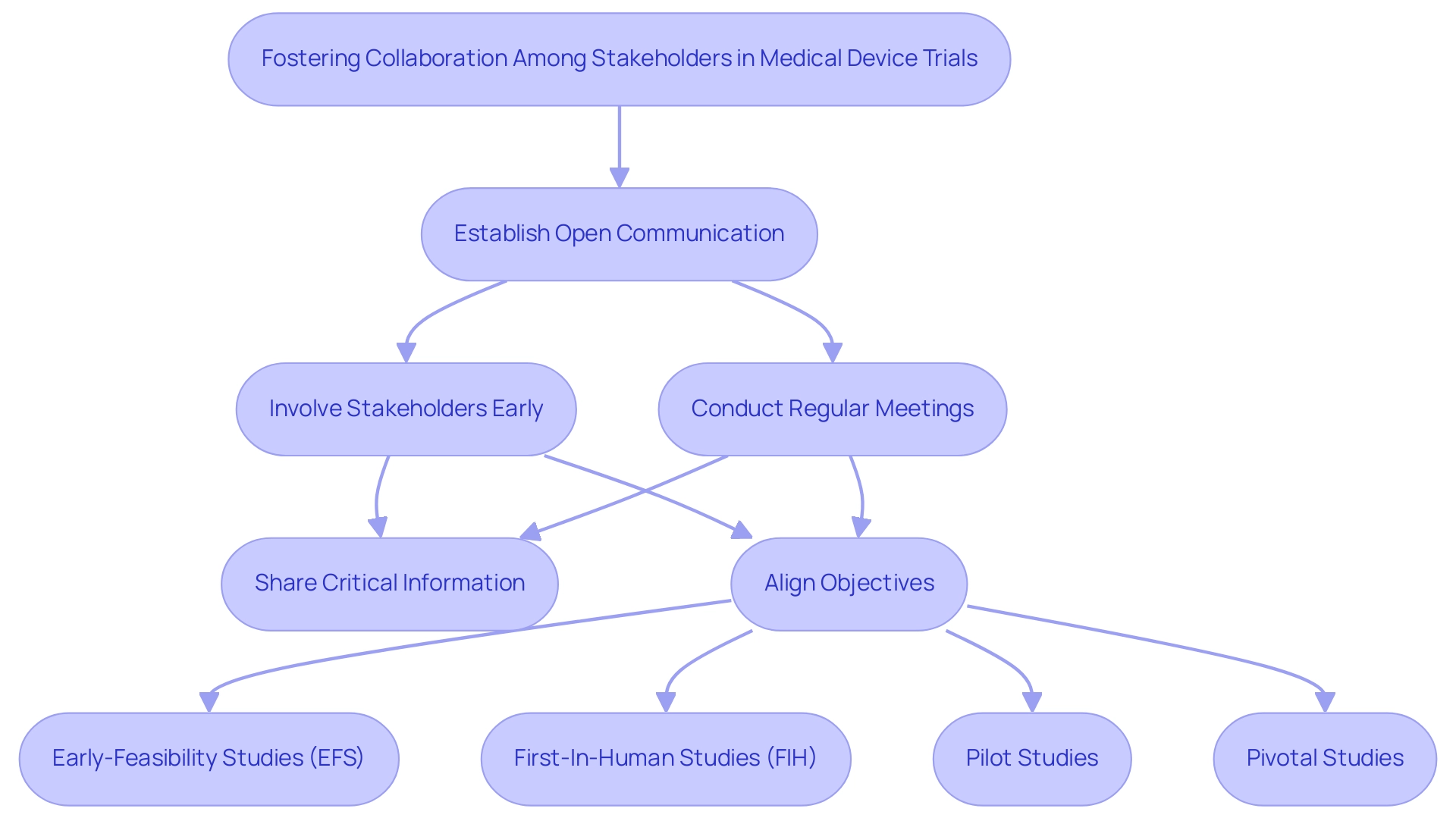
Encouraging cooperation among stakeholders is essential for optimizing medical device trial design in Latin America. Establishing open lines of communication between researchers, sponsors, regulatory bodies like INVIMA, and ethics committees can alleviate misunderstandings and facilitate the sharing of critical information. Regular meetings and updates not only promote transparency but also ensure that all parties are aligned on objectives and methodologies.
Involving stakeholders early in the research design process is crucial for optimizing medical device trial design, as it provides valuable insights that enhance the project's relevance and acceptability. Moreover, incorporating extensive research management services—spanning from feasibility studies, site selection, compliance evaluations, setup, import permits, project oversight, and reporting—establishes strong partnerships founded on trust and mutual benefit. This collaborative approach not only contributes to more efficient and effective evaluations, including:
- Optimizing Medical Device Trial Design for Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
but also amplifies the positive impact on local economies through job creation and improved healthcare outcomes.
Under the leadership of Julio Martinez-Clark, bioaccess advocates for Medtech clinical research in Latin America, further enhancing the credibility and relevance of these efforts.
Continuous Monitoring and Adaptive Trial Designs
Incorporating ongoing observation into medical equipment evaluations is progressively acknowledged as a best practice. By implementing adaptive study designs, researchers can make real-time adjustments based on interim results, which plays a crucial role in optimizing medical device trial design while enhancing patient safety and the study's effectiveness. For instance, if early data suggest that a particular patient group is responding exceptionally well to the device, the study can be adapted to focus on this demographic.
Ongoing monitoring not only permits improved resource allocation by pinpointing underperforming areas that may need intervention but also enhances the comprehensive clinical study management services provided by bioaccess®. Their expertise encompasses:
- Viability assessments
- Site selection
- Compliance evaluations
- Experimental setup
- Import permits
- Project management
- Reporting
With over 20 years of experience in Medtech, bioaccess® has ensured a streamlined approach that has previously achieved significant outcomes, such as a 50% reduction in recruitment time and a 95% retention rate in partnerships like that of GlobalCare Clinical Trials in Colombia.
This flexibility not only enhances the likelihood of success in testing but also contributes to optimizing medical device trial design while aligning with regulatory expectations for patient-centric research.
Post-Trial Considerations and Real-World Evidence Generation
Post-trial factors are a crucial element of the medical instrument lifecycle, particularly regarding expedited research services in Latin America. After finishing a trial, gathering real-world evidence (RWE) through post-market surveillance becomes crucial to monitor the long-term safety and effectiveness of the product. This process includes monitoring patient results, adverse events, and overall equipment performance across various healthcare environments.
By leveraging the expertise of organizations like bioaccess®, which specializes in managing:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
researchers can more effectively establish a systematic approach to gather and analyze RWE. This not only enhances the understanding of the system's impact but also supports regulatory compliance, particularly with INVIMA's oversight as a Level 4 health authority recognized by PAHO/WHO. The challenges faced by Medtech companies in Latin America, such as regulatory hurdles and resource fragmentation, highlight the importance of optimizing medical device trial design to prioritize post-trial evaluations.
This enables researchers to contribute to the ongoing improvement of medical devices, ensuring they continue to meet patient needs while bridging gaps in clinical research and innovation.

Conclusion
The intricacies of medical device trials demand a meticulous and strategic approach to ensure their success. Key design considerations, such as:
- Identifying relevant endpoints
- Determining appropriate sample sizes
- Employing randomization techniques
are fundamental in crafting studies that yield valid and reliable results. Leveraging technology and data further enhances trial design, with innovations like artificial intelligence streamlining patient selection and adaptive methodologies allowing for real-time modifications based on interim findings.
Regulatory compliance remains a cornerstone of effective clinical trials, particularly in the diverse landscape of Latin America. Familiarity with regulations and maintaining rigorous oversight are crucial for protecting patient safety and ensuring the credibility of results. Collaboration among stakeholders, including researchers, sponsors, and regulatory bodies, fosters transparency and shared understanding, ultimately leading to more robust trial designs.
Moreover, the importance of continuous monitoring and post-trial evaluations cannot be overstated. Adaptive trial designs enable researchers to allocate resources efficiently and focus on the most promising patient groups, while post-trial considerations, including the generation of real-world evidence, are vital for assessing long-term device performance and safety.
In conclusion, navigating the complexities of medical device trials requires a comprehensive approach that integrates effective design principles, regulatory adherence, and collaborative efforts. By embracing these strategies, stakeholders can enhance the safety and efficacy of medical devices, ultimately benefiting patient populations and advancing healthcare outcomes in a meaningful way.




