Overview
The article emphasizes best practices for the trial design of Class III medical devices, highlighting the critical nature of comprehensive clinical trials to ensure safety and efficacy prior to market introduction. It underscores that meticulous planning, strict adherence to regulatory requirements, and effective patient recruitment strategies are essential for achieving successful trial outcomes. This is exemplified by the experiences and services provided by organizations like bioaccess®, which play a pivotal role in navigating the complexities of clinical research.
Introduction
In the realm of medical technology, Class III devices stand at the forefront, representing the highest risk category of medical equipment essential for life-sustaining treatments. These sophisticated innovations, including pacemakers and advanced prosthetics, not only enhance patient outcomes but also necessitate rigorous clinical trials to ensure their safety and efficacy.
As the market for these devices continues to expand—projected to reach staggering figures in the billions—the complexity of their development and the evolving regulatory landscape present significant challenges for manufacturers.
This article delves into the critical aspects of Class III medical devices, exploring:
- Their definitions
- The stages of clinical trials
- Best practices in trial design
- Effective patient recruitment strategies
- The importance of post-market surveillance
It highlights the vital role organizations like bioaccess® play in navigating this intricate field.
Understanding Class III Medical Devices: Definitions and Importance
Class III medical equipment represents the highest risk category, encompassing technologies that are vital for sustaining life, preventing impairment, or mitigating significant health risks. Notable examples include:
- Pacemakers
- Implantable defibrillators
- Advanced prosthetic instruments
The intricate nature of these instruments necessitates a trial design for Class III devices that includes comprehensive clinical trials to validate their safety and efficacy prior to market introduction.
The design and functionality of Class III products have profound implications for patient outcomes, underscoring the importance of meticulous research in this domain. Recent statistics indicate that the U.S. market for Class III medical products is projected to range significantly, with estimates varying from 29 to 374 billion dollars. These forecasts are based on historical developments, current trends, and key market indicators, reflecting the growing demand and innovation within this sector. Furthermore, the regulatory landscape is evolving, as evidenced by Johnson & Johnson Services Inc.'s recent approval in China for its MONARCH Platform, a minimally invasive, robot-assisted technology for bronchoscopy.
This development emphasizes the growing significance of Class III instruments in tackling complex medical challenges. The influence of Class III instruments on patient outcomes is not merely theoretical; real-world examples demonstrate their transformative potential. Research studies concentrating on high-risk medical devices have shown considerable enhancements in patient survival rates and quality of life. Organizations like bioaccess® play an essential role in this environment by providing extensive trial design for Class III devices, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project management
With more than 20 years of experience in Medtech, bioaccess®'s expertise in trial design for Class III devices, including managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, is vital for Medtech startups navigating the complexities of research and regulatory requirements, including those established by INVIMA, the Colombian National Food and Drug Surveillance Institute. A notable example is Avantec Vascular's first-in-human clinical study of an innovative vascular instrument in Latin America, supported by bioaccess™, which underscores the importance of strategic partnerships in advancing medical technology. As the landscape of medical technology continues to advance, understanding the nuances of Class III products and their regulatory requirements becomes paramount for researchers aiming to enhance patient care through innovative solutions.
Navigating Regulatory Requirements for Class III Device Trials
Class III medical products encounter the most stringent regulatory scrutiny, primarily under the oversight of the FDA in the United States. To introduce a Class III product to the market, manufacturers must navigate the premarket approval (PMA) process, which entails trial design for Class III devices to validate the product's safety and efficacy. This procedure is not only time-consuming but also complex, necessitating meticulous documentation that encompasses research study protocols, informed consent forms, and comprehensive data analysis plans.
Recent statistics reveal that the average PMA approval timeline for Class III products can extend beyond 300 days, underscoring the complexity of the evaluation process. Furthermore, since October 1, 2007, most establishments are required to pay an establishment registration fee, contributing to the regulatory costs associated with bringing these products to market. For instance, the approval procedure for Class III products frequently involves an exhaustive examination of medical data, which must demonstrate substantial proof of safety and effectiveness.
Successful examples of PMA processes highlight the importance of aligning trial design for Class III devices with FDA regulatory expectations. A notable case involved a Class III device that achieved approval following a well-organized study demonstrating its effectiveness, leading to its successful market introduction.
As of 2025, the FDA continues to enforce stringent regulations for Class III medical devices, emphasizing the necessity for robust empirical evidence. Manufacturers must also recognize the importance of obtaining a CE mark for the European market, which requires a conformity assessment and ongoing monitoring to ensure compliance with EU regulations. This process is vital for market access in Europe and demands careful planning in the trial design for Class III devices.
Moreover, companies can leverage regulatory intelligence services, such as those offered by bioaccess®, to navigate compliance management throughout the PMA process. bioaccess® provides a comprehensive array of services, including feasibility assessments, study preparation, initiation, investigator selection, project oversight, and updates on study progress and adverse occurrences, ensuring that research studies adhere to regulatory standards effectively. By understanding these regulatory requirements and integrating them into the trial design for Class III devices, researchers can mitigate potential approval delays and streamline the pathway to market for innovative medical technologies.
Additionally, bioaccess® is dedicated to safeguarding information security and fostering client trust, with established grievance and data protection procedures to transparently address any concerns. This commitment is crucial for building trust among medical equipment startups as they navigate the challenges of clinical studies in Latin America.
Stages of Clinical Trials for Class III Devices: A Comprehensive Overview
Trial design for Class III devices typically advances through three essential stages: Pilot Studies, Pivotal Studies, and Post-Market Surveillance.
- Pilot Investigations: These initial examinations are vital for evaluating the viability of the research design and collecting preliminary information. They help identify potential challenges and refine methodologies before larger-scale trials commence. Effective reporting of pilot investigations is crucial; guidelines recommend including feasibility objectives, recruitment strategies, and outcome measures to enhance the quality and utility of the findings. Adhering to these guidelines can significantly improve planning for future research. As emphasized in a case analysis on reporting pilot investigations, including specific details can enhance better communication of findings within the research community. Moreover, the insights obtained from pilot experiments can guide later assessments, strengthening the idea that, as Lehana Thabane states, "No examination is a complete failure; it can always be used as a poor example!"
- Crucial Studies: Following successful preliminary investigations, crucial studies are conducted on a larger scale to provide conclusive proof of the apparatus's safety and effectiveness. These assessments are often a prerequisite for the trial design for Class III devices prior to Premarket Approval (PMA) submission. Recent information suggests that the trial design for Class III devices usually includes a considerable number of participants, ensuring strong statistical power to identify significant results. The replication of results from Multi-Regional Clinical Trials (MRCT) is particularly important in this phase, as it establishes the validity of the concept and helps identify any biases. This replication is essential for ensuring that the findings are applicable across diverse populations and settings. For instance, Avantec Vascular's first-in-human clinical trial of an innovative vascular instrument in Latin America, supported by bioaccess™, exemplifies the significance of conducting pivotal trials in this region.
- Post-Market Surveillance: After a product gains approval, post-market surveillance research becomes crucial for observing its performance in the general population. These studies ensure ongoing safety and efficacy, providing critical insights into the technology's long-term impact on patient health. They also assist in the detection of rare adverse events that may not have been evident during earlier study phases.
Each of these stages plays a pivotal role in building a comprehensive understanding of a Class III device's impact on patient health, which is essential for effective trial design for Class III devices, ultimately contributing to enhanced health outcomes and patient safety. As bioaccess® connects innovative medtech firms with the opportunity for carrying out research studies in Latin America, the significance of pilot studies and crucial experiments cannot be emphasized enough; they act as essential components in the research landscape, directing the advancement and enhancement of innovative medical technologies. Moreover, a Cochrane review discovered that across 11 studies of different designs and 328 participants, electromechanical and robot-assisted arm training enhanced strength but did not enhance the use of the arm in daily activities, highlighting the intricacies and results that can emerge from research.
Best Practices in Trial Design: Methodologies for Class III Devices
Optimal approaches in trial design for Class III devices are essential for guaranteeing the reliability and validity of research outcomes. Key elements include:
- Defining clear endpoints
- Selecting appropriate control groups
- Ensuring adequate sample sizes
For instance, recent data indicates that a significant portion of studies, approximately 24.1%, were registered in the Iranian Registry of Clinical Trials (IRCT), with a median target sample size of only 70 patients. This underscores the necessity for researchers to establish robust sample sizes to enhance the statistical power of their studies.
Utilizing randomized controlled experiments is often regarded as the gold standard in clinical research, as it mitigates bias and bolsters the validity of results. Successful examples of trial design for Class III devices demonstrate how rigorous methodologies can yield meaningful insights and facilitate regulatory approval. Furthermore, employing adaptive study designs empowers researchers to respond dynamically to interim results, enabling modifications that can enhance the study's efficiency and relevance.
A patient-centric approach is paramount; study protocols should be crafted with participant needs and safety in mind. This includes well-defined inclusion and exclusion criteria, which are critical for obtaining reliable safety and effectiveness data. A case analysis on trial design for Class III devices illustrates that inclusion and exclusion criteria, such as age, gender, and medical history, must be meticulously considered to ensure a representative sample.
Moreover, it is crucial to acknowledge that long-term endpoint evaluations in research studies may resemble observational studies, complicating interpretation and necessitating careful consideration in study design. Additionally, the interpretation of secondary, exploratory, and subgroup analyses should be approached with caution due to the heightened likelihood of false-positive conclusions. As Robert Peter Gale, a consultant, emphasizes, rigorous study design is vital for obtaining valid results. As medical studies evolve, remaining aware of recent techniques and optimal trial design for Class III devices is essential for researchers aiming to enhance medical technology efficiently.
At bioaccess®, we leverage over 20 years of expertise in managing clinical studies across Latin America, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
to ensure that your clinical research is conducted with the highest standards of quality and compliance.
Effective Patient Recruitment Strategies for Class III Device Trials
Effective patient recruitment strategies for Class III device studies are increasingly reliant on innovative approaches. This includes trial designs that leverage digital platforms, engage healthcare providers, and collaborate with patient advocacy groups. A well-defined and compelling messaging strategy is crucial; it should articulate the goals and potential benefits for participants clearly. Recent studies indicate that employing diverse recruitment methods—such as community outreach and targeted social media campaigns—has significantly improved reach and engagement, leading to higher recruitment success rates.
For instance, a descriptive analysis of early-phase clinical studies revealed that those with robust planning and design experienced notably higher median recruitment rates, particularly in Phase I and Phase I–III studies, which demonstrated superior rates compared to Phase I–II studies. This underscores the necessity of tailoring recruitment strategies to meet the unique needs of potential participants. Flexibility in study design, including accommodating patient schedules and providing transportation assistance, can further enhance participation rates.
Moreover, successful digital outreach initiatives have proven effective in connecting with potential participants. Engaging with patient advocacy groups not only fosters trust but also amplifies outreach efforts, as these organizations often have established networks that facilitate recruitment. Alberto M. Borobia from La Paz University Hospital noted, "Pediatric research has shown exceptionally favorable recruitment rates, in contrast to what has been reported by other analyses," emphasizing the significance of understanding various demographics in recruitment strategies.
Additionally, statistically significant subgroup differences identified in research related to participant recruitment success highlight the importance of tailored recruitment strategies.
In this context, bioaccess® offers extensive research management services encompassing feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. With over 20 years of expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess® is well-positioned to enhance recruitment strategies and ensure the success of trial designs for Class III devices. As the landscape of clinical research transforms, employing these effective patient recruitment strategies, supported by the capabilities of bioaccess®, will be crucial for trial design for Class III devices, advancing their success while contributing to local economies through job creation and healthcare enhancement.
Data Management and Analysis: Ensuring Integrity in Class III Device Trials
Effective data management and analysis are critical in the trial design for Class III devices, ensuring data integrity and compliance with regulatory standards. The implementation of electronic data capture (EDC) systems is essential; these systems facilitate real-time data entry and monitoring, significantly enhancing the efficiency of data collection processes. Researchers must develop comprehensive data management plans that clearly outline data collection methodologies, storage protocols, and analysis techniques tailored to the specific requirements of trial design for Class III devices.
In this context, bioaccess offers a wide array of comprehensive research management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting on serious and non-serious adverse events
- Review and feedback on research documents
These services are designed to assist researchers in navigating the complexities of medical studies, ensuring adherence to regulatory standards, and enhancing study outcomes.
Regular audits and quality checks are vital in identifying and addressing discrepancies within the data, thereby safeguarding the integrity of the results. A recent survey revealed that the personnel responsible for reviewing data quality reports typically include:
- Chief investigators
- Auditors
- Data managers
- Sponsors
This highlights the collaborative effort required to uphold data standards.
Moreover, employing appropriate statistical analysis methods is crucial for the reliability of findings. A solid understanding of statistical concepts among researchers in the health field can prevent misinterpretation of data and ensure the proper application of statistical methods, which is essential for the integrity of health studies. This importance is underscored in a case study examining how insufficient training in biostatistics can compromise study integrity.
Additionally, it is noteworthy that the benefit of intensive onsite source data verification (SDV) was found to be minimal at 8.2% compared to risk-based monitoring, emphasizing the necessity for efficient data management practices. As Manisha Desai, a Professor of Medicine, stated, "We’ve learned that the FDA and regulatory agencies can be quite responsive... and get rid of things that might be unnecessary." This insight underscores the importance of adapting to regulatory expectations while maintaining data integrity.
As the landscape of clinical research evolves, staying abreast of current trends in EDC systems and data management protocols will further enhance the quality and compliance of trial design for Class III devices.
Post-Market Surveillance: Monitoring Class III Devices After Approval
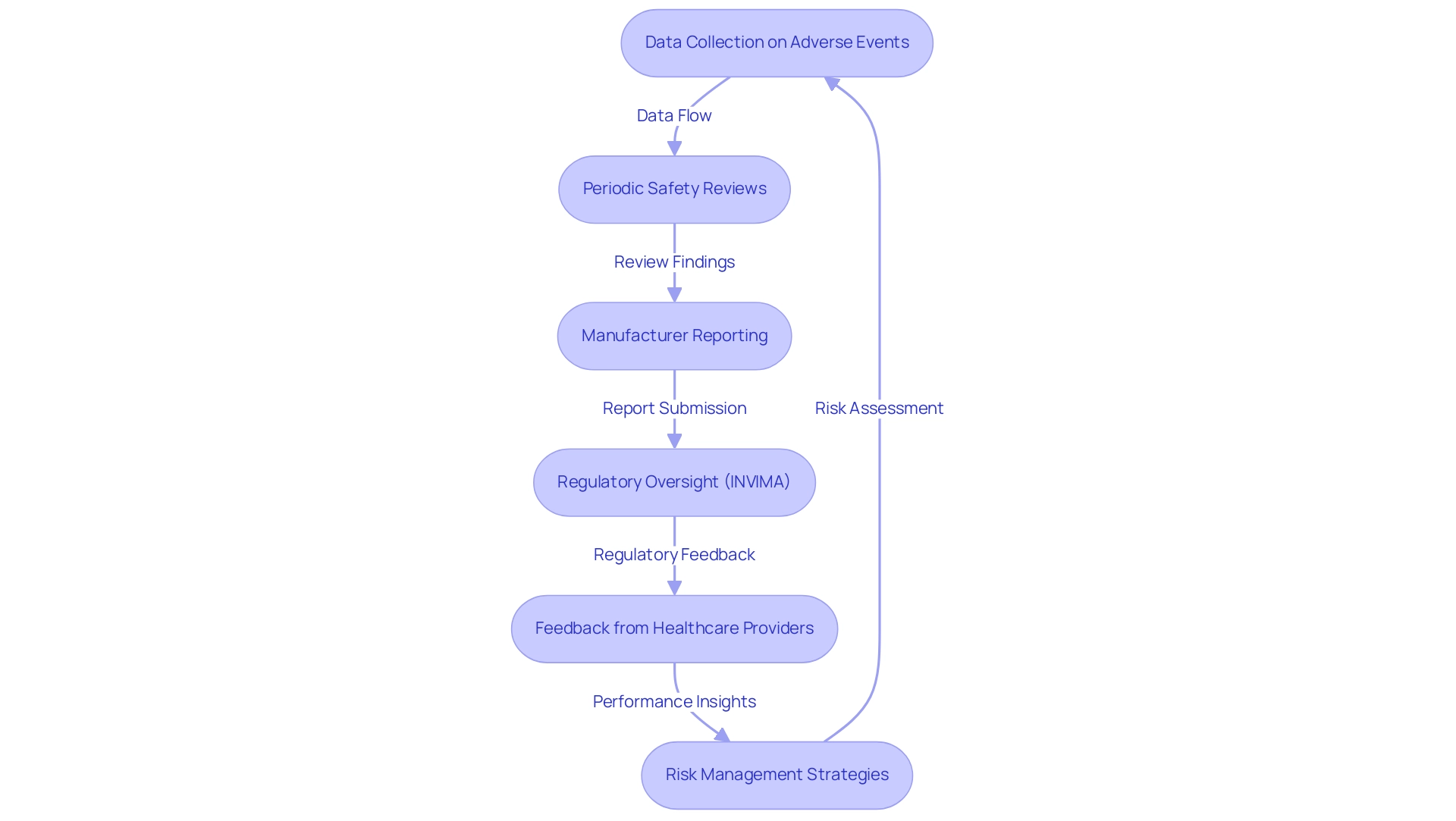
Post-market surveillance for Class III products is a critical process that necessitates systematic monitoring of their performance following approval for use. This comprehensive approach encompasses the collection of data on adverse events, conducting periodic safety reviews, and implementing robust risk management strategies. Manufacturers are typically mandated to submit detailed post-market surveillance reports to regulatory authorities, such as INVIMA (Colombia National Food and Drug Surveillance Institute), which oversees the marketing and manufacturing of health products in Colombia.
INVIMA's role is crucial in ensuring compliance with health standards and implementing best practices, thereby safeguarding patient health.
With over 15 years of experience in the Medtech sector, bioaccess® comprehends the complexities involved in post-market surveillance. Recent studies underscore the necessity for more proactive post-market surveillance mechanisms to enhance the detection of emerging safety issues. For instance, serious adverse events were reported in only 14.6% of cases, with a notable 4,108 hospitalizations documented, underscoring the need for improved reporting practices.
As noted by Sanket S Dhruva, an associate professor, "Patient safety concerns may not be identified in a timely manner due in part to late manufacturer reporting of medical equipment adverse events." Involving healthcare providers and patients to collect feedback on performance is essential, as it offers valuable insights that can guide ongoing safety evaluations and potential improvements.
Furthermore, the constraints of passive post-market monitoring systems have been highlighted in several case studies, which support the incorporation of unique identifiers into health records. This integration would facilitate a more comprehensive assessment of medical equipment safety, allowing for timely identification of patient safety concerns. The reliance on manufacturers for reporting adverse events can lead to delays in addressing critical safety issues, emphasizing the importance of active surveillance strategies in safeguarding patient health.
For manufacturers and regulatory authorities like INVIMA, these findings highlight the need for improved collaboration and proactive actions to ensure the safety and efficacy of medical products in the market. Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, stresses that comprehending these regulatory frameworks is essential for successful research and innovation in the Medtech sector.
Future Trends in Trial Design for Class III Medical Devices
Future trends in trial design for Class III devices are set to be significantly influenced by technological advancements and evolving regulatory landscapes. The integration of digital health technologies, such as remote monitoring and telehealth, is anticipated to enhance patient engagement and streamline data collection processes. Remarkably, approximately 80 percent of medical studies now employ Electronic Data Capture (EDC) systems, with an increasing number of locations adopting electronic patient-reported outcomes (ePRO) and electronic consent (eConsent) methods.
In addition, just under 20 percent of research locations utilize Resource, with two-thirds of surveyed sites considering it very or extremely beneficial. This shift underscores the growing reliance on digital solutions to enhance research efficiency and participant experience.
In this context, bioaccess® stands out as a leader in providing expedited medical device research services in Latin America, leveraging over 20 years of expertise in managing Early-Feasibility Evaluations (EFS), First-In-Human Evaluations (FIH), Pilot Evaluations, Pivotal Evaluations, and Post-Market Follow-Up Evaluations (PMCF). Their comprehensive clinical study management services encompass feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, ensuring that studies are conducted efficiently and effectively. bioaccess® adopts a customized approach tailored to the unique needs of each client, thereby enhancing the overall experience.
Adaptive designs are also gaining prominence, empowering researchers to make real-time modifications based on interim results. This flexibility not only optimizes resource allocation but also accelerates the path to market for innovative devices. For instance, the All of Us study exemplifies the successful application of mobile technology in large-scale health research, demonstrating how comprehensive data collection can inform precision medicine.
Moreover, the focus on patient-centric approaches is reshaping study designs, ensuring that the needs and preferences of participants are prioritized throughout the research process. Predictive digital tools are providing insights into disease progression and therapy response, guiding healthcare provider decision-making within the context of study designs. As Bloss and colleagues noted, there is a pressing need to redesign the current human research protection system to better align with these advancements.
As the landscape evolves, industry experts predict that these trends will continue to drive innovation in trial design for Class III devices, ultimately enhancing the quality and relevance of clinical research. bioaccess® is well-positioned as a vetted CRO and consulting partner for U.S. medical device companies in Colombia.
Conclusion
Class III medical devices are indispensable in modern healthcare, representing the highest risk category of medical equipment crucial for life-sustaining treatments. The complexities inherent in their development demand rigorous clinical trials, which encompass stages from pilot studies to pivotal trials and post-market surveillance. Each phase is essential for ensuring safety and efficacy, with best practices in trial design significantly enhancing the reliability of outcomes.
The significance of effective patient recruitment strategies cannot be overstated, as they directly influence the success of clinical trials. By leveraging innovative approaches and engaging with diverse communities, manufacturers can optimize participant enrollment, thereby enhancing the overall quality of research. Moreover, robust data management and analysis practices are vital for maintaining the integrity of trial results, ensuring compliance with regulatory standards, and fostering trust among stakeholders.
As the medical technology landscape continues to evolve, organizations like bioaccess® play a pivotal role in navigating the complexities of regulatory requirements and trial design. Their expertise in clinical trial management, particularly in Latin America, positions them as invaluable partners for Medtech companies striving to bring innovative solutions to market.
Looking ahead, embracing future trends such as digital health technologies and adaptive trial designs will be essential for enhancing patient engagement and streamlining processes. By prioritizing patient-centric approaches and harnessing technological advancements, the medical device industry can continue to drive forward innovations that significantly improve patient care and health outcomes.
Frequently Asked Questions
What is Class III medical equipment?
Class III medical equipment represents the highest risk category of medical devices, which are essential for sustaining life, preventing impairment, or mitigating significant health risks. Examples include pacemakers, implantable defibrillators, and advanced prosthetic instruments.
Why are clinical trials important for Class III devices?
Clinical trials are crucial for Class III devices to validate their safety and efficacy before they can be introduced to the market. The intricate nature of these instruments necessitates comprehensive research to ensure patient outcomes are positively impacted.
What is the projected market size for Class III medical products in the U.S.?
The U.S. market for Class III medical products is projected to range from 29 to 374 billion dollars, reflecting growing demand and innovation in this sector.
What role does bioaccess® play in the development of Class III devices?
bioaccess® provides extensive trial design services for Class III devices, including feasibility assessments, site selection, compliance evaluations, and project management, helping Medtech startups navigate research and regulatory complexities.
What is the premarket approval (PMA) process for Class III products?
The PMA process is a rigorous regulatory pathway required for introducing Class III products to the market, involving trial design to validate safety and efficacy, meticulous documentation, and an average approval timeline that can extend beyond 300 days.
What are the regulatory requirements for Class III devices in the U.S.?
Class III devices undergo stringent regulatory scrutiny primarily by the FDA, requiring substantial proof of safety and effectiveness through exhaustive examination of medical data and compliance with detailed documentation.
What is the significance of obtaining a CE mark for Class III devices?
Obtaining a CE mark is essential for market access in Europe, requiring a conformity assessment and ongoing compliance monitoring with EU regulations, which demands careful planning in trial design.
How can companies ensure compliance during the PMA process?
Companies can leverage regulatory intelligence services, such as those offered by bioaccess®, which assist with compliance management throughout the PMA process, including feasibility assessments, study preparation, and project oversight.
What commitment does bioaccess® have regarding information security?
bioaccess® is dedicated to safeguarding information security and fostering client trust, with established grievance and data protection procedures to transparently address any concerns raised by clients.




