Overview
Best practices for trial design involving wearable devices in clinical research are crucial for enhancing the effectiveness and efficiency of clinical trials. These practices encompass:
- The selection of user-friendly technology
- The establishment of robust data management protocols
- The incorporation of participant feedback mechanisms
Such strategies not only foster improved participant engagement but also streamline data collection processes, thereby ensuring data integrity and compliance. In a landscape where clinical research continually evolves, these considerations are paramount for successful outcomes.
Introduction
The integration of wearable devices into clinical trials signifies a pivotal transformation in medical research, presenting unparalleled opportunities for real-time health monitoring and data collection.
As researchers increasingly embrace smart technology to bolster participant engagement and enhance data accuracy, the clinical trial landscape is evolving at an unprecedented pace.
Projections indicate substantial growth in the wearable device market, underscoring profound implications for patient outcomes and personalized treatment.
This article delves into the transformative role of wearables in clinical research, exploring their:
- Advantages
- Regulatory considerations
- Best practices for integration
- Future trends poised to redefine the field
The Transformative Role of Wearable Devices in Clinical Trials
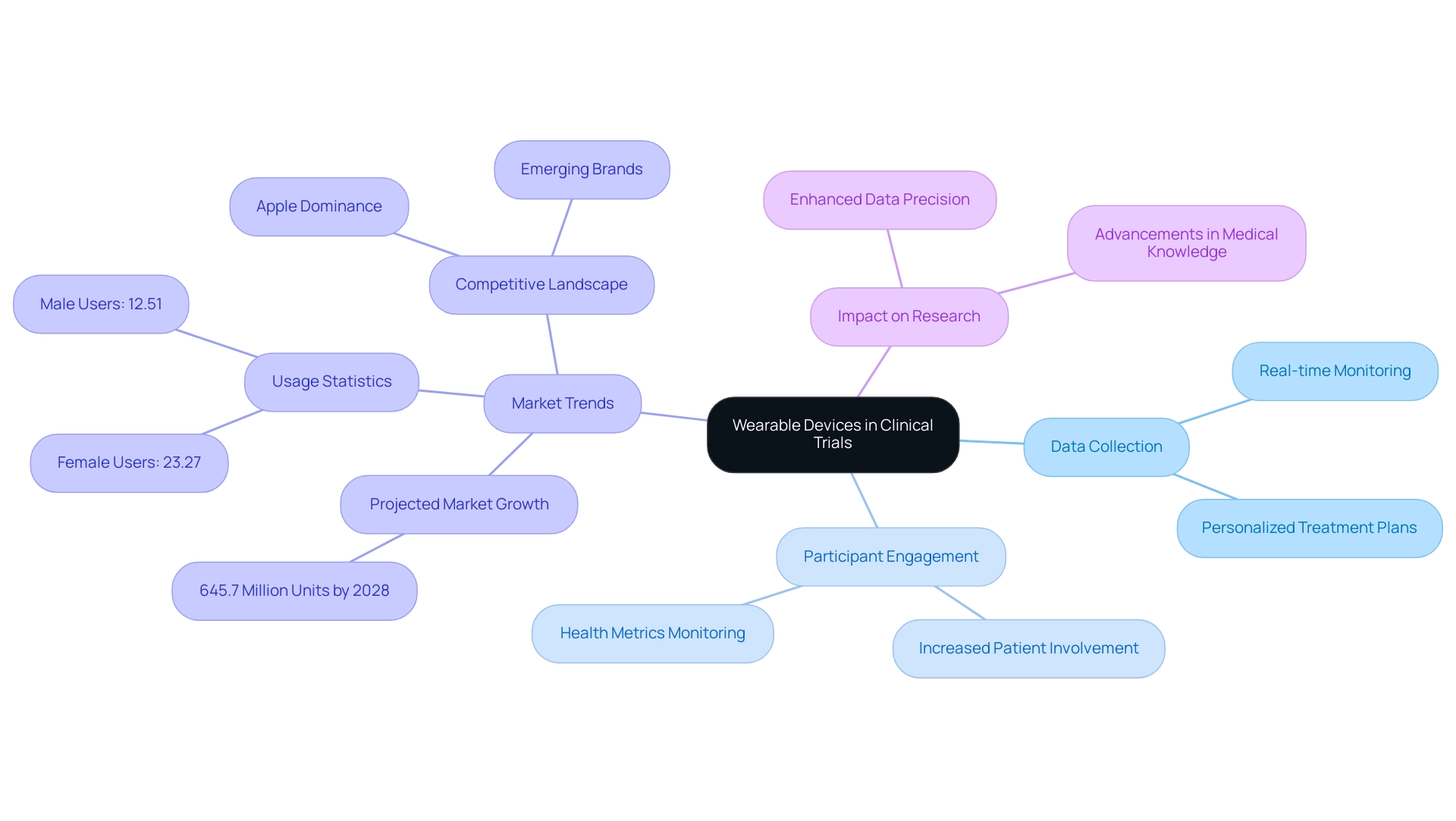
Wearable gadgets are swiftly becoming vital elements of research studies, aiding in the ongoing observation of participants' health metrics and facilitating real-time data gathering. This transition from conventional methods to portable technology empowers researchers to collect more extensive data, significantly enhancing the quality of medical research. For instance, gadgets like smartwatches and fitness trackers can monitor vital signs, activity levels, and medication compliance, providing a comprehensive view of patient health during the study.
The influence of body-mounted technology on clinical trial data collection is profound, particularly when considering trial design for wearable devices. Recent statistics indicate that the global personal technology market is projected to reach 645.7 million units by 2028, driven by rising consumer interest in health and fitness monitoring. Notably, a survey revealed that 23.27% of female participants and 12.51% of male participants utilized a healthcare gadget in the previous year, underscoring the increasing acceptance and integration of these technologies into daily life.
Furthermore, the market for wearable tech has experienced significant trends from 2019 to 2023, with established brands like Apple leading in shipments while new entrants carve out their niches. This competitive landscape underscores the growing importance of portable devices in research, as they not only enhance data precision but also increase patient engagement. Participants can actively monitor their health metrics, fostering a more meaningful contribution to the research process.
As Tajammul Pangarkar, CMO at Prudour Pvt Ltd, observes, "The incorporation of smart technology in medical trials is not merely a trend; it is a revolutionary method that improves data gathering and patient participation."
The advantages of portable technology extend beyond data collection; they also facilitate the development of personalized treatment plans, ultimately improving patient outcomes and advancing medical knowledge. As emphasized in the case study 'Wearable Technology Market Trends (2019-2023)', understanding the competitive dynamics within the market is crucial for comprehending how these innovations can be applied in research settings. As the landscape of medical studies continues to evolve, the trial design for wearable devices will be pivotal in shaping the future of trial frameworks and methodologies.
Advantages of Integrating Wearable Technology in Clinical Research
Incorporating technology into clinical research presents numerous advantages, such as enhanced data accuracy, improved patient compliance, and reduced operational costs. Wearable devices facilitate the collection of objective data, significantly reducing the reliance on self-reported measures that are often subject to bias and inaccuracies. Recent findings reveal that adherence rates among patients using devices can reach between 70% and 80%, representing a substantial improvement particularly beneficial in managing chronic diseases.
Ongoing observation through these devices not only promotes improved health outcomes but also empowers patients to engage actively in their health management. Moreover, the use of such technology streamlines data gathering procedures, diminishing the need for regular in-person appointments. This efficiency enhances management processes while alleviating the overall burden on participants, thereby fostering higher compliance rates.
For instance, during the COVID-19 pandemic, the surge in telemedicine adoption led to a notable increase in the utilization of monitoring technology for remote patient observation. This shift underscored the critical role of smart devices in modern healthcare, especially regarding trial design for wearable devices, as they track vital signs, physical activity, and sleep patterns, rendering them indispensable tools in clinical trials.
As the landscape of clinical research evolves, the trial design for wearable devices is set to redefine patient engagement and data collection methodologies, ultimately resulting in more effective and efficient clinical trials. Looking ahead, it is anticipated that by 2030, approximately 40% of practices will integrate artificial intelligence into remote patient monitoring systems, further enhancing the functionality of smart technology. Additionally, the recent investment of $20 million by MediSense for a device aimed at continuous monitoring of respiratory conditions highlights the growing interest and financial backing in this sector.
Furthermore, with 63% of women monitoring their fertility and 67% tracking their menstrual cycle using devices, it is evident that health tracking is becoming increasingly prevalent among consumers. As Tajammul Pangarkar, CMO at Prudour Pvt Ltd, notes, the incorporation of such technologies is essential for enhancing research and improving patient outcomes.
Navigating the Regulatory Landscape for Wearable Devices
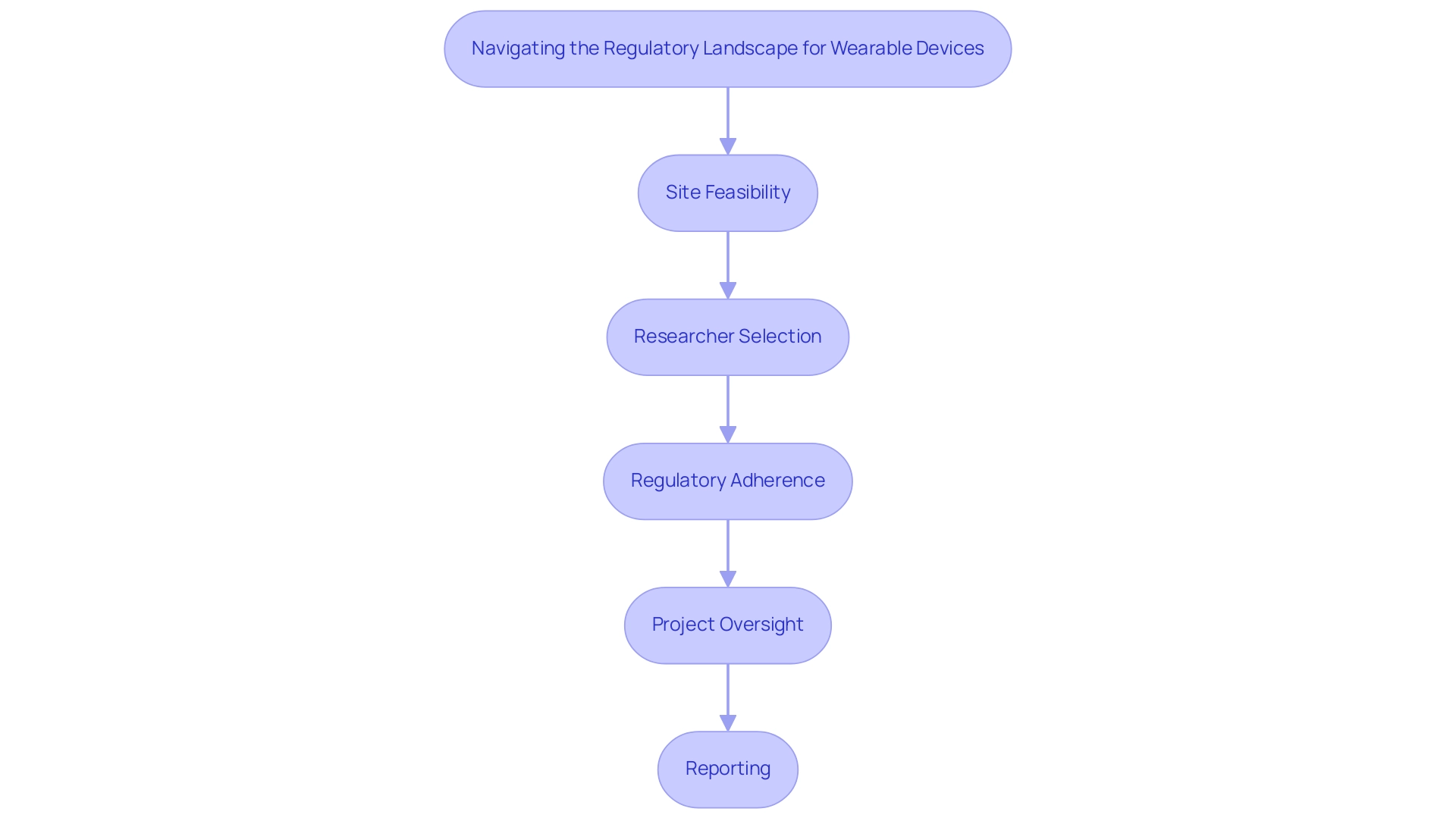
Navigating the regulatory environment for wearable technology in clinical studies necessitates a comprehensive understanding of the guidelines established by regulatory authorities such as the FDA, EMA, and INVIMA (Colombia National Food and Drug Surveillance Institute). Founded in 1992 under Colombia's Ministry of Health and Social Protection, INVIMA plays a vital role in inspecting and overseeing the marketing and production of health products, including medical equipment. As a Level 4 health authority recognized by the Pan American Health Organization/World Health Organization, INVIMA ensures compliance with safety and efficacy standards, which may require researchers to engage in pre-market submissions or conduct post-market surveillance.
Early engagement with INVIMA and other regulatory bodies during the study design phase is crucial to clarify requirements and mitigate potential delays. This is particularly important for medical startups facing challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints. The thorough procedure for progressing medical apparatus studies encompasses:
- Site feasibility
- Researcher selection
- Regulatory adherence
- Project oversight
- Reporting
All of which are vital for successful results.
Bioaccess offers specific services in these areas, including trial set-up and project management, to support researchers in navigating these complexities. As the landscape of digital health technologies evolves, staying informed about the latest regulations is essential for researchers. This knowledge not only affects the approval process but also shapes the practical use of smart technology in healthcare environments. For instance, in 2021, other vendors shipped 199.9 million units, highlighting the growing market for wearable devices and the relevance of regulatory compliance in this context.
Recent collaborations among global pharmaceutical sponsors have led to the development of digital health technologies (DHTs) that serve as medical endpoints, showcasing a proactive approach to regulatory compliance. Moreover, expert insights from Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasize the importance of evaluating key factors when integrating wearable-derived measures into healthcare programs. By addressing regulatory considerations early and continuously throughout the research process, clinical investigators can facilitate smoother execution and bolster stakeholder confidence in the outcomes of their studies. This strategic approach not only boosts the credibility of the research but also aligns with the increasing trend of incorporating innovative trial design for wearable devices that utilize technology worn on the body.
Additionally, sponsors must evaluate key factors to optimize the utility of device data and ensure data integrity, further reinforcing the importance of collaboration in regulatory compliance, as illustrated by the case study on 'Collaboration for Digital Endpoint Development,' which highlights how partnerships can enhance the development of effective digital health solutions.
Best Practices for Optimizing Wearable Device Integration in Clinical Trials
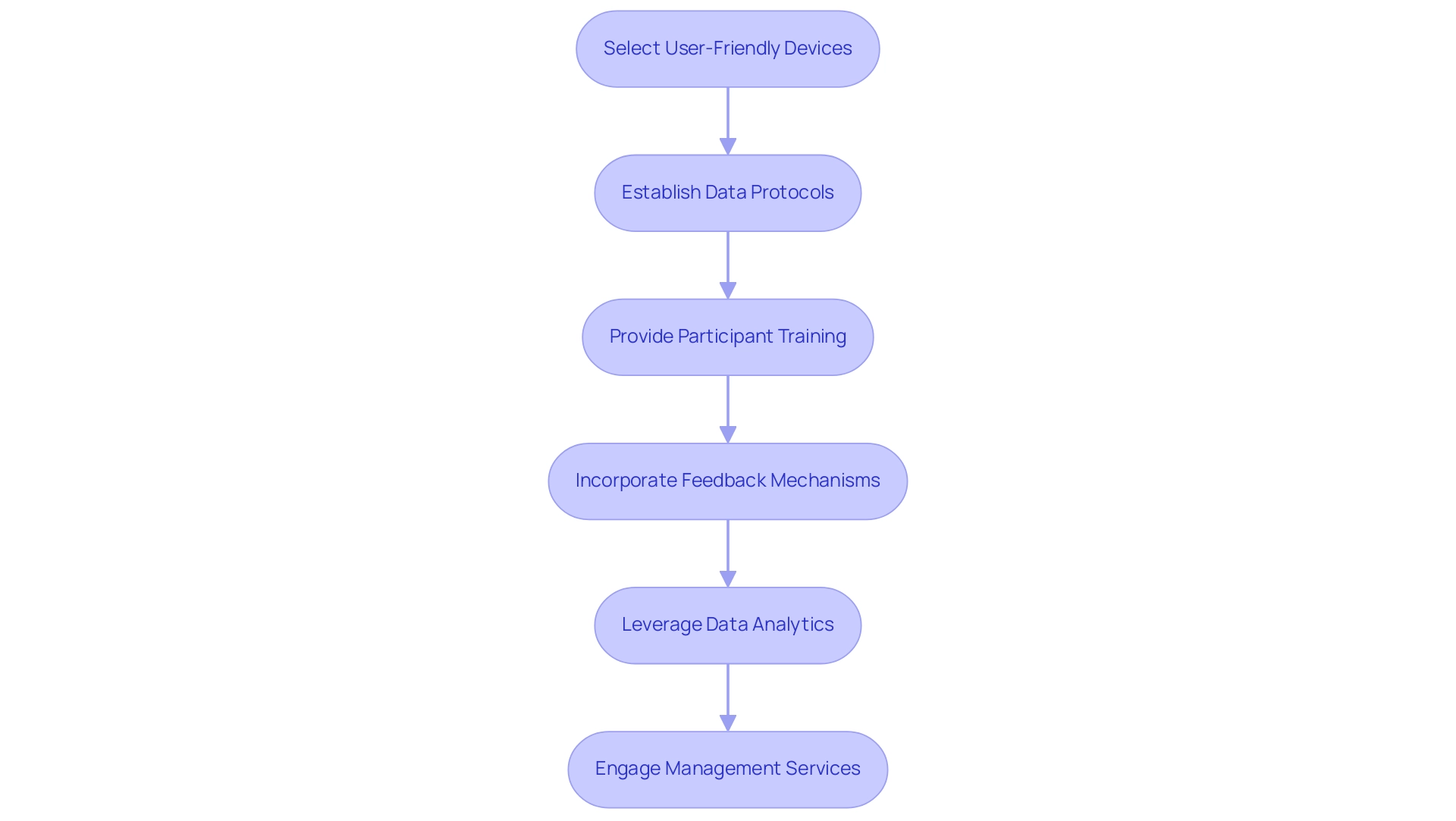
To effectively incorporate wearable technology in clinical studies, researchers must adhere to best practices that enhance trial design for wearable devices, ultimately improving participant involvement and data integrity.
Firstly, selecting user-friendly and comfortable tools is essential; studies indicate that this choice can elevate user compliance rates by 15-25%. When participants feel at ease with the technology, their adherence to study protocols is significantly influenced.
Secondly, establishing robust protocols for data collection and management is critical. This includes secure transmission and storage of data, which safeguards participant information and upholds the integrity of the study. Comprehensive training for participants on equipment usage further boosts compliance and ensures high-quality data collection.
Incorporating feedback mechanisms is another vital practice. Allowing participants to share their experiences with the equipment enables researchers to identify and address potential issues early in the testing process. This proactive strategy not only enhances participant satisfaction but also contributes to the overall success of the study.
The FDA's Patient-Focused Drug Development program and structured approaches like patient advisory boards can be particularly effective in this context.
Moreover, leveraging data analytics tools facilitates real-time monitoring and analysis of collected data. This capability empowers researchers to make timely adjustments to the study, optimizing the use of portable technology and enhancing overall research results.
In addition to these practices, extensive management services for studies, such as those provided by bioaccess, are crucial for the successful integration of wearable technology. From feasibility studies and site selection to compliance reviews and setup, bioaccess meticulously manages all aspects of the study. Furthermore, acquiring essential import permits and nationalizing investigational devices, along with efficient project management and reporting on study status and adverse events, plays a significant role in the smooth execution of studies, ultimately impacting local economies through job creation and healthcare improvements.
Real-world examples underscore the effectiveness of these strategies. For instance, a case study titled 'Prioritizing Data and Platform Stability' highlights the importance of preserving data integrity in research studies, especially amid rapid technological advancements. By selecting platforms that prioritize stability and backward compatibility, as noted by Christine Guo, PhD, researchers can ensure consistent data quality throughout the study, even if it necessitates delaying the adoption of the latest technology.
In summary, by focusing on user-friendly devices, clear data protocols, participant training, feedback mechanisms, and real-time data analytics, alongside comprehensive trial management services from bioaccess, researchers can significantly enhance the trial design for wearable devices, ultimately leading to more successful outcomes.
Future Trends in Wearable Technology for Clinical Research
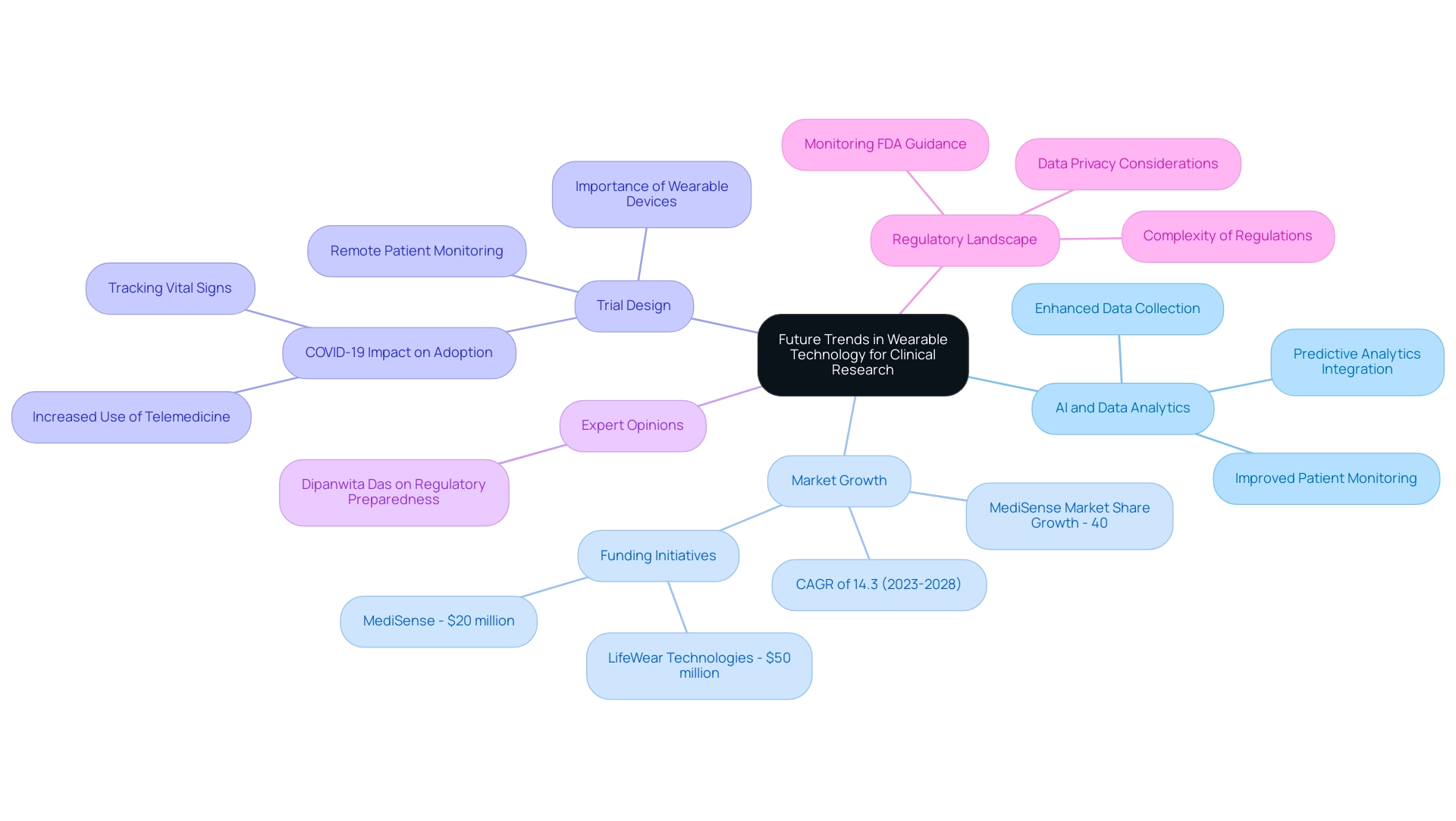
The future of portable technology in medical research is set to undergo significant transformations, primarily propelled by advancements in artificial intelligence (AI), machine learning, and data analytics. These innovations are paving the way for more sophisticated data collection methods, enabling the integration of real-time health metrics with predictive analytics. Such integration not only enhances patient monitoring but also markedly improves health outcomes.
Dr. Sergio Alvarado, Clinical Trial Manager at bioaccess®, firmly believes that innovation and medical research are paramount for making a significant impact in the medical field. His focus on artificial intelligence as a diagnostic tool exemplifies the potential of these technologies in enhancing portable devices. Prior to his tenure at bioaccess™, Dr. Alvarado served in the medical affairs department at Novartis and holds an M.D. degree from Universidad de Los Andes, a prestigious institution in Latin America. The global device technology market is projected to expand at a compound annual growth rate (CAGR) of 14.3% from 2023 to 2028, reflecting the increasing demand for these devices in healthcare settings. As decentralized clinical studies gain momentum, the design of trials involving wearable devices becomes increasingly critical, facilitating remote patient monitoring and seamless data collection.
This paradigm shift not only enhances data accuracy but also elevates patient engagement and overall trial efficiency. Recent case studies effectively illustrate this trend. For instance, the COVID-19 pandemic accelerated the acceptance of telemedicine, leading to a surge in the use of devices for remote patient monitoring, particularly those tracking vital signs and physical activity. This evolution underscores the importance of trial design for wearable devices in modern healthcare, establishing these devices as essential tools for health researchers.
Moreover, the influence of AI and machine learning on portable devices is profound. These technologies are refining data collection processes, yielding more nuanced insights into patient health. As researchers delve deeper into the capabilities of wearables, grasping trial design for these devices will be crucial for leveraging wearable technology effectively in research studies.
Expert opinions suggest that the integration of AI-driven analytics will not only enhance data management but also provide deeper insights into patient behavior and treatment effectiveness, ultimately leading to more successful research outcomes.
As Dipanwita Das, CEO & co-founder, emphasizes, "Last, but certainly not the least is regulatory preparedness. Regulations are becoming increasingly intricate and prescriptive and more demanding, but monitoring FDA guidance on novel study designs and DCT approaches, staying updated with international regulations, ensures a very smooth commercialization process, as well as data privacy." This highlights the necessity for researchers in the medical field to adeptly navigate the evolving regulatory landscape.
Furthermore, recent funding initiatives in the technology sector for wearables, such as LifeWear Technologies securing $50 million to enhance product development and MediSense obtaining $20 million to support research for a device monitoring respiratory conditions, underscore the growing significance of these innovations in medical research. MediSense anticipates a 40% market share growth, further illustrating the competitive landscape and potential impact of trial design for wearable devices in clinical trials.
Conclusion
The integration of wearable devices into clinical trials signifies a pivotal advancement in medical research, enhancing participant engagement and ensuring data accuracy. By facilitating continuous health monitoring and real-time data collection, wearables offer a comprehensive view of patient health, significantly elevating the quality of clinical studies. As the wearable device market expands, the potential for personalized treatment and improved patient outcomes becomes increasingly evident.
Successfully navigating the regulatory landscape is paramount for effective wearable integration in trials. Early collaboration with regulatory authorities mitigates delays and bolsters confidence in research findings, thereby streamlining the trial process.
Implementing best practices, such as choosing user-friendly devices and establishing robust data management protocols, is essential for optimizing trial outcomes. These strategies not only enhance compliance rates but also guarantee reliable data collection. Furthermore, real-time analytics and participant feedback mechanisms significantly amplify research effectiveness.
Looking forward, advancements in artificial intelligence and data analytics are poised to revolutionize the role of wearables in clinical research. These innovations will facilitate more sophisticated data collection and predictive analytics, ultimately improving patient monitoring and clinical outcomes.
In conclusion, the utilization of wearable devices in clinical trials represents a transformative shift in medical research. By leveraging these technologies, researchers can enhance data collection, engage patients more effectively, and ultimately drive better health outcomes, paving the way for a more personalized approach to clinical studies.
Frequently Asked Questions
How are wearable gadgets impacting medical research?
Wearable gadgets are becoming essential in medical research by enabling real-time data collection and ongoing observation of participants' health metrics, which enhances the quality of research.
What types of health metrics can wearable devices monitor?
Wearable devices like smartwatches and fitness trackers can monitor vital signs, activity levels, and medication compliance, providing a comprehensive view of patient health during studies.
What is the projected growth of the personal technology market related to wearable devices?
The global personal technology market is projected to reach 645.7 million units by 2028, driven by increasing consumer interest in health and fitness monitoring.
What percentage of participants used healthcare gadgets in the previous year?
A survey indicated that 23.27% of female participants and 12.51% of male participants used a healthcare gadget in the previous year.
How do wearable devices enhance patient engagement in clinical trials?
Wearable devices allow participants to actively monitor their health metrics, fostering a more meaningful contribution to the research process.
What are some advantages of incorporating wearable technology into clinical research?
Advantages include enhanced data accuracy, improved patient compliance, and reduced operational costs, as wearable devices provide objective data and reduce reliance on self-reported measures.
What are the adherence rates among patients using wearable devices?
Adherence rates among patients using wearable devices can reach between 70% and 80%, particularly benefiting the management of chronic diseases.
How did the COVID-19 pandemic influence the use of monitoring technology?
The pandemic led to a surge in telemedicine adoption and increased utilization of monitoring technology for remote patient observation, highlighting the critical role of smart devices in healthcare.
What future trends are expected in the integration of technology in clinical research?
By 2030, it is anticipated that approximately 40% of practices will integrate artificial intelligence into remote patient monitoring systems, further enhancing the functionality of smart technology.
What recent investment highlights the growing interest in wearable technology for health monitoring?
MediSense recently invested $20 million in a device aimed at continuous monitoring of respiratory conditions, indicating significant financial backing in the wearable tech sector.




